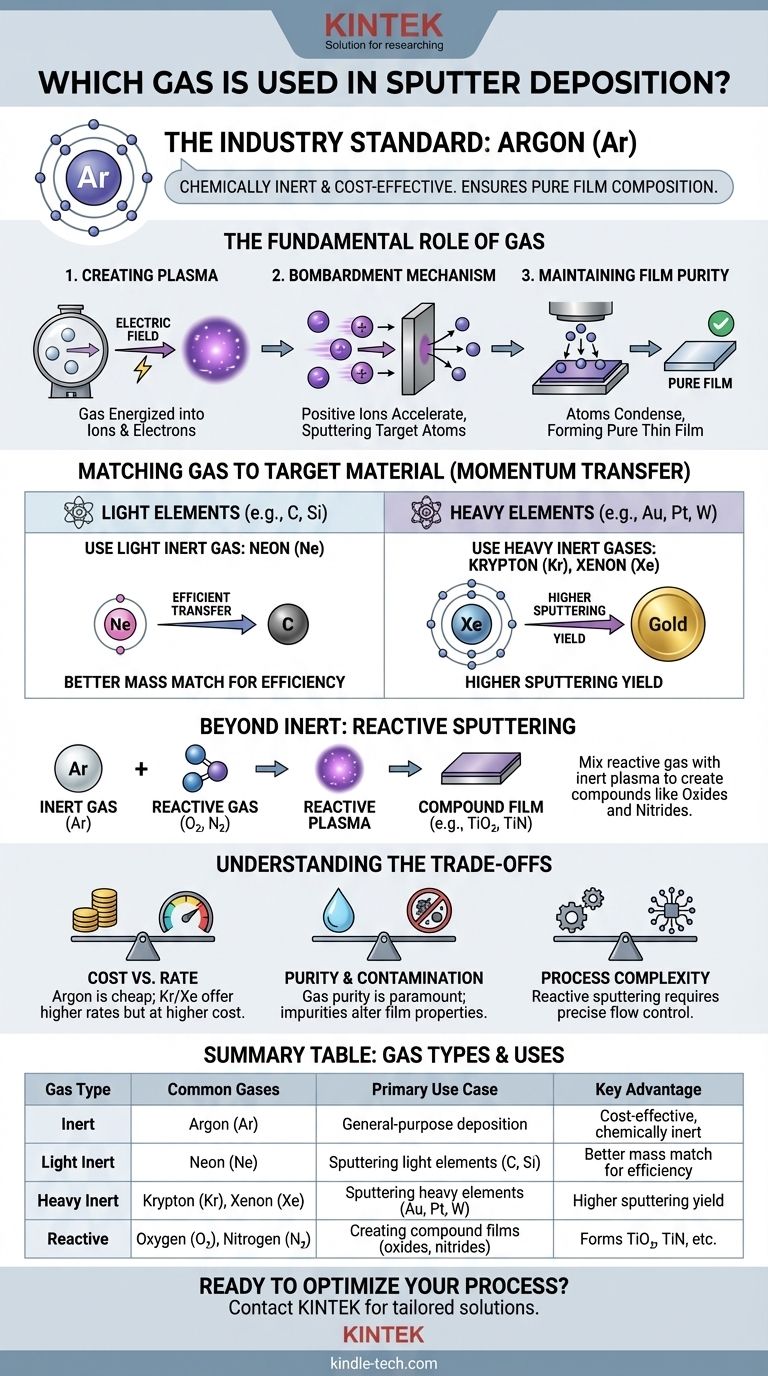
In sputter deposition, the most commonly used gas is Argon (Ar). This is because Argon is a noble gas, meaning it is chemically inert and will not react with the target material during the process. This allows for the deposition of a thin film that has the same pure composition as the source material.
The choice of gas in sputter deposition is a critical engineering decision. While Argon is the default for its inert nature and cost-effectiveness, the ideal gas is selected based on a trade-off between sputtering efficiency, cost, and the desired final film composition.

The Fundamental Role of Gas in Sputtering
To understand why specific gases are chosen, we must first understand the role the gas plays in the deposition process itself. The gas is not merely a background environment; it is the active medium that drives the entire sputtering mechanism.
Creating the Plasma
Sputter deposition begins by introducing a low-pressure gas into a vacuum chamber. An electric field is then applied, which energizes the gas and transforms it into a plasma—a state of matter consisting of positive ions and free electrons.
The Bombardment Mechanism
These newly created positive gas ions are accelerated by the electric field and directed towards the "target," which is a solid block of the material you wish to deposit. The ions bombard the target with high energy, physically knocking off, or sputtering, atoms from its surface.
Maintaining Film Purity
These sputtered atoms then travel through the chamber and condense onto a substrate (like a silicon wafer or glass slide), forming a thin, uniform film. Using an inert gas like Argon is crucial for ensuring the deposited film is pure and has the same chemical makeup as the target.
Matching the Gas to the Target Material
While Argon is the workhorse of sputtering, process efficiency can be significantly improved by matching the gas to the target. This decision is governed by a core principle of physics.
The Principle of Momentum Transfer
Think of the process like a game of pool. For the most efficient transfer of energy and momentum, the mass of the colliding objects should be similar. The same is true in sputtering: maximum sputtering occurs when the mass of the gas ion is close to the mass of the target atom.
Sputtering Light Elements
When sputtering lighter target materials (like carbon or silicon), a lighter inert gas is more efficient. Neon (Ne), while more expensive than Argon, provides a better mass match and can increase the sputtering rate.
Sputtering Heavy Elements
Conversely, for heavy target materials (like gold, platinum, or tungsten), heavier inert gases are far more effective. Krypton (Kr) and Xenon (Xe) have a much higher atomic mass than Argon, leading to a dramatic increase in sputtering efficiency for these heavy elements.
Beyond Inert: The Power of Reactive Sputtering
In some applications, the goal is not to deposit a pure material but to create a compound. This is achieved through a process called reactive sputtering, where the gas is intentionally chosen to react with the sputtered material.
The Goal: Depositing Compounds
In reactive sputtering, a reactive gas (like Oxygen or Nitrogen) is mixed into the primary inert gas (usually Argon). As atoms are sputtered from the target, they react with this gas to form a new compound.
Creating Oxides and Nitrides
This is the standard method for producing technologically important films. For example, sputtering a titanium target in a mix of Argon and Oxygen will deposit a titanium dioxide (TiO₂) film. Sputtering the same target in Argon and Nitrogen will create a hard titanium nitride (TiN) coating.
Where the Reaction Occurs
Depending on the process parameters, this chemical reaction can take place on the surface of the target, in-flight as the atoms travel to the substrate, or directly on the substrate itself.
Understanding the Trade-offs
Selecting the right gas is always a balance of competing factors.
Cost vs. Sputtering Rate
Argon is abundant and inexpensive, making it the default choice. Neon, Krypton, and especially Xenon are significantly more expensive. You must weigh the higher cost against the potential gains in process speed and efficiency.
Purity and Contamination
The purity of the sputtering gas is paramount. Any impurities, such as water vapor or oxygen, in your inert gas supply can become inadvertently incorporated into your film, altering its electrical or optical properties.
Process Complexity
Reactive sputtering is a powerful but complex process. Controlling the gas mixture and reaction chemistry to achieve the desired film stoichiometry requires precise control over gas flow rates and pumping speeds.
Selecting the Right Gas for Your Application
Your choice of gas is determined entirely by your project's technical and economic goals.
- If your primary focus is general-purpose, cost-effective thin film deposition: Stick with Argon, as it provides the best balance of performance and cost for a wide range of materials.
- If your primary focus is maximizing sputtering rate for a specific material: Match the ion mass to the target atom mass—use Neon for light elements and Krypton or Xenon for heavy elements if the budget allows.
- If your primary focus is creating a compound film like an oxide or nitride: Use a reactive sputtering process by introducing a gas like Oxygen or Nitrogen into your Argon plasma.
Ultimately, the gas you choose is a fundamental parameter that defines both the efficiency of your process and the properties of the final material you create.
Summary Table:
| Gas Type | Common Gases | Primary Use Case | Key Advantage |
|---|---|---|---|
| Inert | Argon (Ar) | General-purpose deposition | Cost-effective, chemically inert |
| Light Inert | Neon (Ne) | Sputtering light elements (C, Si) | Better mass match for efficiency |
| Heavy Inert | Krypton (Kr), Xenon (Xe) | Sputtering heavy elements (Au, Pt, W) | Higher sputtering yield |
| Reactive | Oxygen (O₂), Nitrogen (N₂) | Creating compound films (oxides, nitrides) | Forms TiO₂, TiN, etc. |
Ready to optimize your sputter deposition process? The right gas choice is critical for achieving high-quality, efficient thin films. At KINTEK, we specialize in providing laboratory equipment and consumables tailored to your specific research and production needs. Whether you're working with inert gases for pure metal deposition or reactive gases for advanced compound films, our expertise can help you maximize sputtering efficiency and film quality. Contact our experts today to discuss how we can support your lab's unique requirements with precision equipment and consumables.
Visual Guide
Related Products
- CVD Diamond Dressing Tools for Precision Applications
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Custom PTFE Teflon Parts Manufacturer F4 Conical Flask Triangular Flask 50 100 250ml
- Custom PTFE Teflon Parts Manufacturer for Three-Necked Round Bottom Flask
- Lab Plastic PVC Calender Stretch Film Casting Machine for Film Testing
People Also Ask
- What are the advantages of the CVD diamond growing process compared to the HPHT process? Master Precision & Efficiency
- What are diamond coated tools used for? Conquer Abrasive Materials with Superior Tool Life
- What is the application of diamond coating? Solve Complex Wear, Heat, and Corrosion Problems
- What are some ethical issues with diamond mining? Uncover the Hidden Costs of Your Gemstone
- How thick is CVD diamond coating? Balancing Durability and Stress for Optimal Performance








