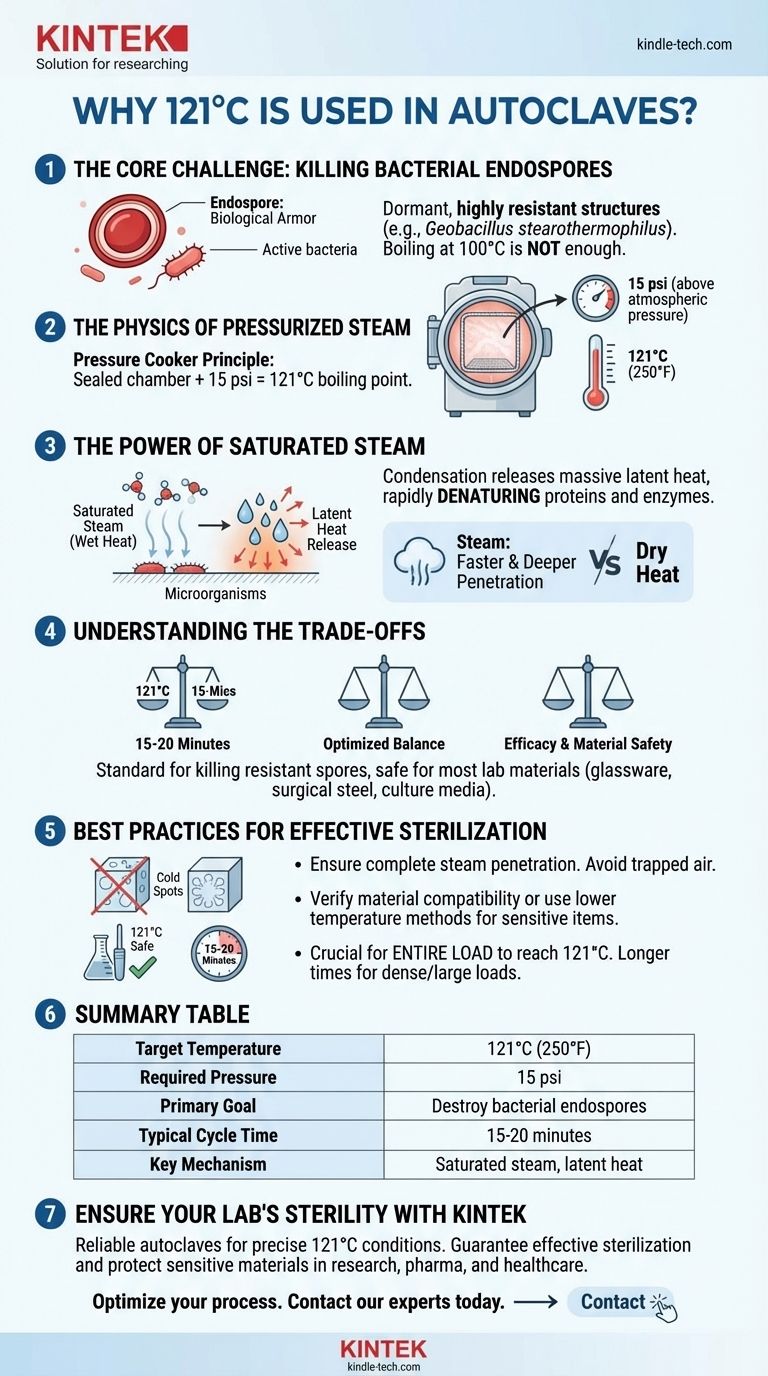
The standard temperature for autoclaving is 121°C because it is the scientifically validated temperature required to reliably destroy the most heat-resistant microorganisms known, specifically bacterial endospores. This temperature is achieved by using pressurized steam, which is far more effective at sterilization than dry heat or boiling water alone.
The core principle is simple: increasing the pressure inside the autoclave to 15 psi above atmospheric pressure raises the boiling point of water from 100°C to 121°C. This creates saturated steam that efficiently transfers lethal heat, guaranteeing the destruction of even the toughest bacterial spores within a practical timeframe of 15-20 minutes.

The Core Challenge: Killing Bacterial Endospores
What Are Endospores?
Endospores are dormant, non-reproductive structures produced by certain bacteria. They are like biological armor, designed to survive extreme conditions of heat, radiation, chemicals, and desiccation that would kill the active (vegetative) bacterium.
The bacterium Geobacillus stearothermophilus is a key example. Its spores are exceptionally heat-resistant and are used as a biological indicator to validate that an autoclave cycle has been successful.
Why Normal Boiling Isn't Enough
Boiling water at 100°C (212°F) is sufficient to kill most vegetative bacteria and viruses. However, it cannot reliably destroy hardy bacterial endospores.
To achieve true sterility—the complete absence of all viable microorganisms—a more powerful method is required.
The Physics of Pressurized Steam
The Temperature-Pressure Relationship
The effectiveness of an autoclave relies on a fundamental law of physics: as you increase the pressure in a sealed chamber, the boiling point of water rises.
The autoclave is essentially a sophisticated pressure cooker. By preventing steam from escaping, it builds pressure internally.
Reaching the Target: 15 psi
At a pressure of 15 pounds per square inch (psi) above normal atmospheric pressure, the boiling point of water is precisely 121°C (250°F).
This creates the ideal environment for sterilization. The high temperature itself is lethal, but the way that heat is delivered is what makes the process so efficient.
The Power of Saturated Steam
The key to autoclaving is "wet heat." At 121°C, the pressurized steam is saturated with thermal energy.
When this steam makes contact with cooler items inside the autoclave, it condenses back into water. This phase change releases a massive amount of latent heat directly onto the surface of the microorganisms, rapidly denaturing their essential proteins and enzymes and causing irreversible cellular damage.
This process is significantly faster and more effective at penetrating dense materials than dry heat at the same temperature.
Understanding the Trade-offs and Best Practices
The Risk of Incomplete Sterilization
The most common failure in autoclaving is not allowing for complete steam penetration. If air is trapped in the chamber or within a load, it creates "cold spots" that will not reach 121°C.
Proper loading, allowing space between items, is critical. For certain loads, especially those with hollow spaces, a vacuum cycle is necessary to remove all air before the steam is introduced.
Balancing Efficacy and Material Damage
While higher temperatures (e.g., 134°C) can sterilize faster, they require higher pressures and can damage sensitive materials.
The 121°C standard is an optimized balance. It is highly effective against the most resistant spores while being safe for a wide range of laboratory materials, including glassware, surgical steel, and certain high-grade plastics and culture media.
The Critical Role of Time
Temperature is only half of the equation. The standard cycle of 121°C for 15 minutes refers to the time the entire load is held at that target temperature.
Larger or denser loads, such as a large flask of liquid or a tightly wrapped instrument pack, require longer cycle times to ensure the center of the load reaches 121°C and is held there for the required duration.
Making the Right Choice for Your Sterilization Goal
To ensure effective sterilization, you must consider both the standard parameters and the nature of your specific load.
- If your primary focus is routine lab media and glassware: The standard 121°C for 15-20 minutes is the proven and reliable gold standard for most applications.
- If your primary focus is sterilizing large liquid volumes or dense waste: You must increase the cycle time significantly to ensure the core of the load reaches temperature and is held there for the full duration.
- If your primary focus is heat-sensitive instruments or plastics: You must verify that your materials are autoclavable at 121°C or consider alternative, lower-temperature sterilization methods.
Understanding the science behind 121°C transforms autoclaving from a routine task into a controlled, reliable process for guaranteed sterility.
Summary Table:
| Key Aspect | Details |
|---|---|
| Target Temperature | 121°C (250°F) |
| Required Pressure | 15 psi above atmospheric pressure |
| Primary Goal | Destroy heat-resistant bacterial endospores |
| Typical Cycle Time | 15-20 minutes (adjustable for load size) |
| Key Mechanism | Saturated steam transfers latent heat efficiently |
Ensure your lab's sterility with reliable autoclaves from KINTEK.
Choosing the right sterilization equipment is critical for eliminating even the most resistant bacterial spores. KINTEK specializes in high-performance lab equipment, including autoclaves designed to maintain precise 121°C conditions for guaranteed results. Our solutions help laboratories in pharmaceuticals, research, and healthcare achieve consistent, effective sterilization while protecting sensitive materials.
Let us help you optimize your sterilization process. Contact our experts today to discuss your lab's specific needs and discover the right autoclave solution for you.
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
People Also Ask
- How does an autoclave ensure the reliability of experimental results? Achieving a Sterile Baseline for Lab Research
- How is an autoclave utilized in antimicrobial experiments? Ensure Precise Nanoparticle Research Integrity
- How should solid materials in bags be prepared for decontamination? Master Steam Penetration for Safe Sterilization
- What experimental conditions do stainless steel autoclaves provide for PCT-A leaching? Optimize Phosphate Glass Testing
- Why is a 24-hour hydrothermal treatment in an autoclave necessary for BMO nanosheets? Unlock Superior Photocatalysis



















