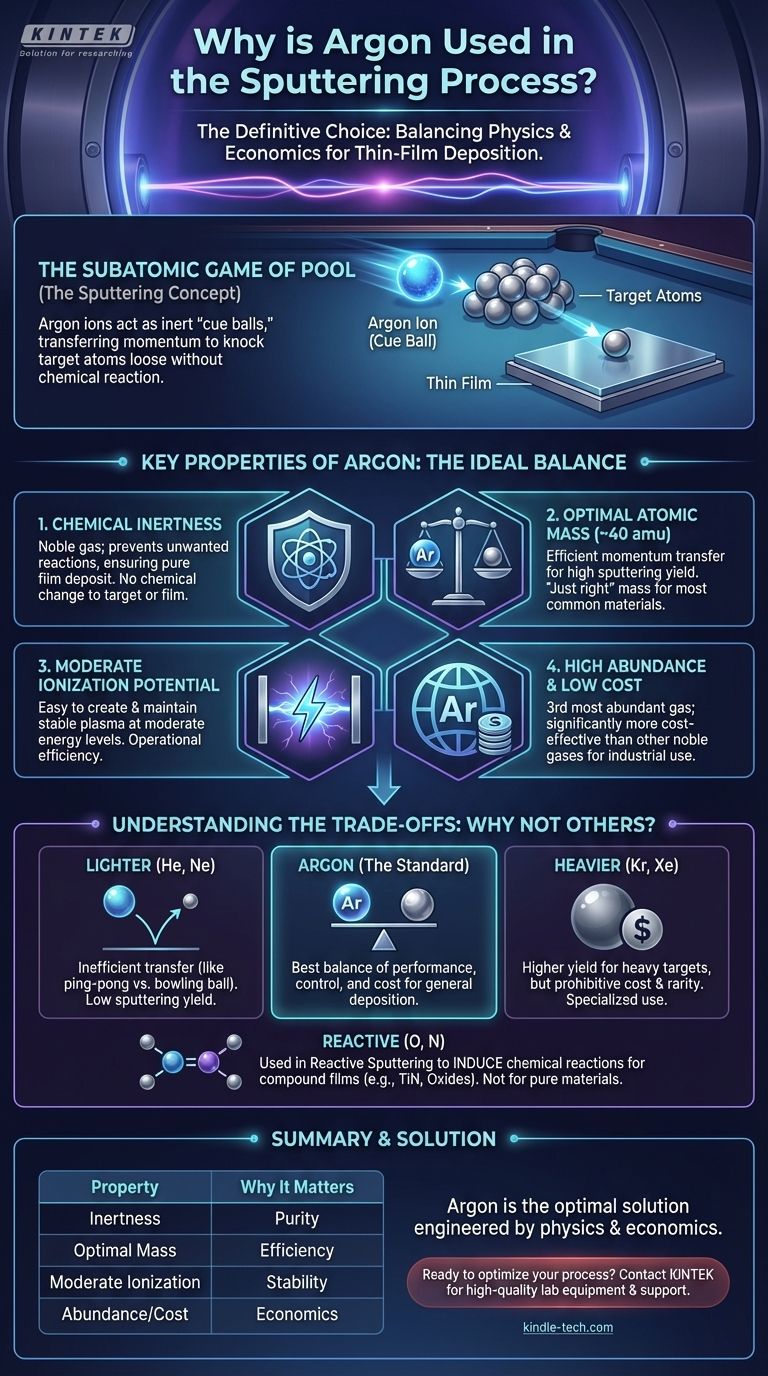
The definitive reason argon is used in sputtering is its unique combination of physical and economic properties. It is a chemically inert noble gas with a high atomic mass, a suitable ionization potential, and it is significantly more abundant and cost-effective than other gases that share these traits.
Sputtering is fundamentally a game of momentum transfer, like a subatomic game of pool. The goal is to choose a "cue ball" (an ion) that is inert, cost-effective, and has just the right mass to efficiently knock the target atoms loose without causing unwanted chemical reactions. For the vast majority of applications, argon is the perfect compromise.

The Role of Gas in the Sputtering Process
To understand why argon is the standard, we must first understand the fundamental role of gas in creating the sputtering effect. The process gas is not just a background environment; it is the active medium that drives the entire deposition.
The Need for Plasma
The process begins by introducing a low-pressure gas into a vacuum chamber. A high voltage is then applied, which strips electrons from the gas atoms.
This creates a plasma, an energized state of matter consisting of positive ions (the gas atoms that lost an electron) and free electrons.
The Mission: Physical Momentum Transfer
These newly formed positive ions are accelerated by an electric field, causing them to slam into the surface of the "target" material you wish to deposit.
This collision is a purely physical event. The ion's momentum is transferred to the target atoms, and if the impact is energetic enough, it knocks a target atom loose. This ejected atom then travels through the chamber and deposits as a thin film on your substrate.
Key Properties That Make Argon the Ideal Choice
Argon's dominance comes from its ability to perform the role of the energetic ion better than almost any other gas, especially when balancing performance with cost.
Inert Nature: Preventing Chemical Reactions
Sputtering is a Physical Vapor Deposition (PVD) process. The goal is to physically move atoms from the target to the substrate without changing their chemical nature.
As a noble gas, argon is chemically inert. It will not react with the target material or the growing film, ensuring the deposited layer remains pure.
Optimal Atomic Mass: Efficient Sputtering Yield
The efficiency of sputtering depends heavily on the mass ratio between the incoming ion and the target atom. Think of it like a billiard ball collision.
An argon ion (atomic mass ~40 amu) has enough mass to effectively dislodge atoms from most common metals and materials used in industry (e.g., Titanium, Copper, Aluminum). A lighter ion would bounce off, while a much heavier one might implant itself. Argon strikes an excellent balance, leading to a high sputtering yield—the number of atoms ejected per incoming ion.
Sufficient Ionization Potential: Stable Plasma
Argon ionizes at a relatively moderate energy level (15.76 eV). This means it's easy to create and sustain a stable, high-density argon plasma without requiring extreme power supplies.
This operational ease makes the process repeatable, controllable, and efficient, which is critical in both research and high-volume manufacturing environments.
Abundance and Cost: The Economic Factor
While other gases might offer marginal performance benefits in niche cases, they come at a steep cost. Argon is the third most abundant gas in Earth's atmosphere (~0.93%).
This natural abundance makes it far less expensive to produce and purify than other noble gases like krypton or xenon, making it the only economically viable choice for the vast majority of industrial applications.
Understanding the Trade-offs: Why Not Other Gases?
Choosing argon becomes even clearer when you consider the drawbacks of the alternatives.
Lighter Noble Gases (Helium, Neon)
Helium and Neon are also inert, but their atomic masses are too low. Sending a helium ion at a tungsten target is like throwing a ping-pong ball at a bowling ball; the momentum transfer is extremely inefficient, resulting in a very low sputtering yield.
Heavier Noble Gases (Krypton, Xenon)
Krypton and Xenon are heavier than argon and can actually provide a higher sputtering yield for very heavy target materials. However, they are orders of magnitude rarer and more expensive than argon. Their use is restricted to highly specialized applications where maximum deposition rate is worth the prohibitive cost.
Reactive Gases (Oxygen, Nitrogen)
Gases like oxygen and nitrogen are intentionally introduced in a process called reactive sputtering. Here, the goal is to form a compound film. For example, sputtering a titanium target in a mix of argon and nitrogen creates a hard, gold-colored Titanium Nitride (TiN) film.
These gases are used to induce a chemical reaction, which is fundamentally different from the inert, physical process for which standard argon sputtering is used.
Matching the Gas to Your Sputtering Goal
Your choice of process gas is dictated entirely by the film you intend to create and your operational constraints.
- If your primary focus is cost-effective, general-purpose deposition of pure materials: Argon is the undisputed industry standard, offering the best balance of performance, control, and cost.
- If your primary focus is maximizing sputtering rate for heavy target materials (e.g., gold, platinum): You might consider Krypton or Xenon, but only if the significant increase in cost is justified by the application.
- If your primary focus is creating a specific compound film (like an oxide, nitride, or carbide): You will use reactive sputtering, introducing a precise amount of a reactive gas like oxygen or nitrogen alongside argon.
Ultimately, understanding the properties of argon reveals why it's not just a random choice, but the optimal solution engineered by physics and economics for thin-film deposition.
Summary Table:
| Property | Why It Matters for Sputtering |
|---|---|
| Chemical Inertness | Prevents unwanted reactions, ensuring a pure film deposit. |
| Optimal Atomic Mass (~40 amu) | Enables efficient momentum transfer for a high sputtering yield. |
| Moderate Ionization Potential | Allows for easy creation and maintenance of a stable plasma. |
| High Abundance & Low Cost | Makes it the most economically viable choice for industrial use. |
Ready to optimize your thin-film deposition process? The right equipment is key to leveraging the benefits of argon sputtering. KINTEK specializes in high-quality lab equipment and consumables, providing reliable sputtering systems and expert support to meet your laboratory's specific needs. Contact us today to discuss how we can enhance your research or production capabilities!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Aluminum Foil Current Collector for Lithium Battery
- Custom PTFE Teflon Parts Manufacturer for Non-Standard Insulator Customization
- Platinum Auxiliary Electrode for Laboratory Use
People Also Ask
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- What are the advantages of PECVD? Enable Low-Temperature, High-Quality Thin-Film Deposition
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition















