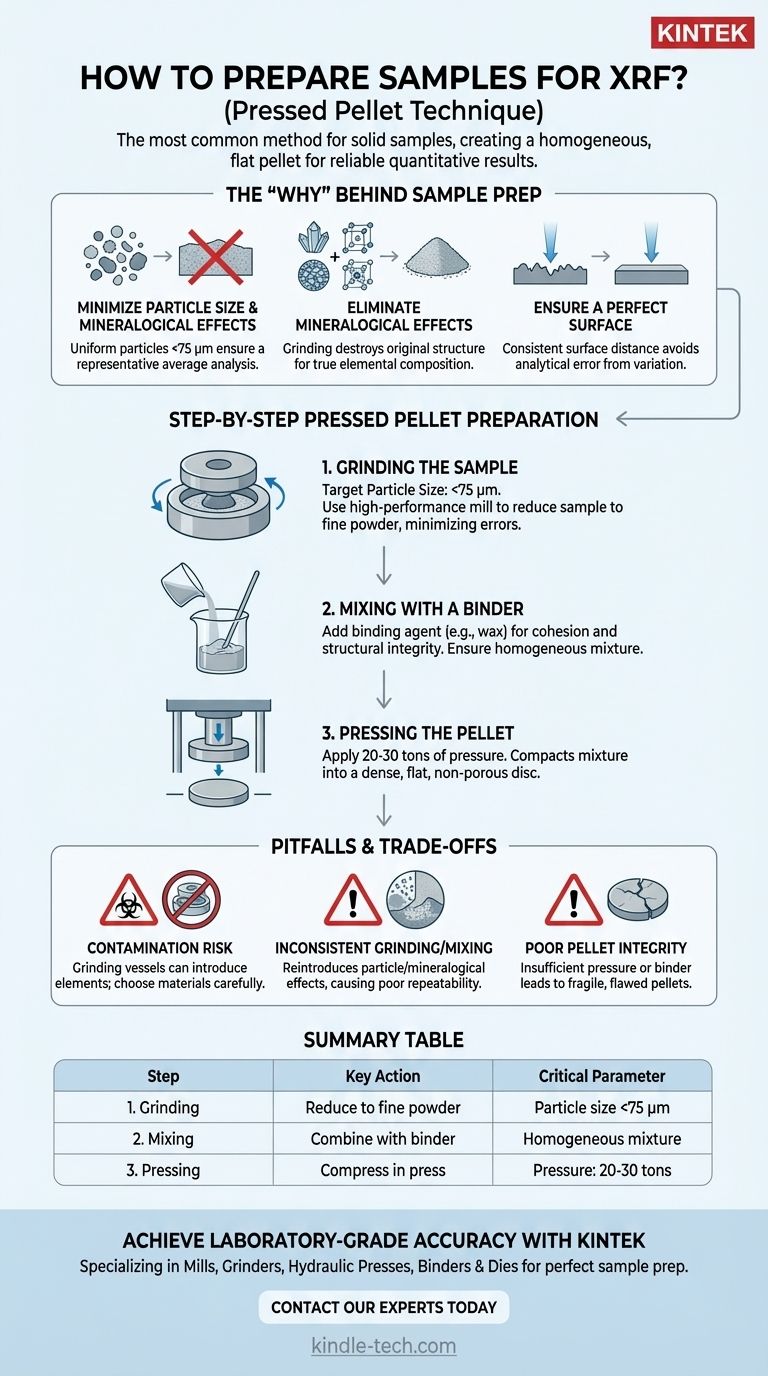
The most common method for preparing solid samples for XRF analysis is the pressed pellet technique. This process involves grinding a sample into a fine, uniform powder, mixing it with a binding agent, and compressing this mixture under high pressure to form a dense, flat pellet suitable for analysis.
The ultimate goal of sample preparation is not just to create a sample, but to produce a perfectly homogeneous and representative surface for the X-ray beam. This single step is the most critical factor in achieving accurate and repeatable analytical results.

The "Why" Behind Meticulous Sample Preparation
X-ray Fluorescence (XRF) is a surface-sensitive analytical technique. The primary X-ray beam interacts with a very shallow layer of the sample, meaning any imperfections on that surface can dramatically skew the results.
Minimizing Particle Size Effects
If a sample contains large, non-uniform particles, the X-ray beam may disproportionately interact with certain elements or minerals. Grinding the sample to a fine powder, ideally with a grain size of less than 75 micrometers (μm), ensures the beam analyzes a statistically representative average of the material.
Eliminating Mineralogical Effects
Different minerals within a sample can absorb or emit X-rays differently, even if they contain the same elements. By grinding the sample into a fine, homogeneous powder, you effectively destroy the original mineral structure, ensuring the analysis reflects the true bulk elemental composition.
Ensuring a Perfect Surface
A rough, cracked, or porous sample surface creates inconsistencies in the distance between the sample and the spectrometer's detector. This variation alters the intensity of the measured fluorescent X-rays, leading to significant analytical error.
Step-by-Step Guide to Pressed Pellet Preparation
The pressed pellet method is the industry standard for achieving high-quality quantitative results from solid materials like rocks, cements, and minerals.
Step 1: Grinding the Sample
The first and most crucial step is to reduce the sample to a fine powder. This is typically done with a high-performance mill, such as a puck-and-ring or planetary ball mill. The target particle size of <75 μm is essential for minimizing analytical errors.
Step 2: Mixing with a Binder
Once ground, the fine powder is mixed with a small amount of a binding agent. This agent, often a wax or cellulosic material, serves two purposes: it acts as a grinding aid to prevent clumping and provides the structural integrity needed to form a durable pellet.
Step 3: Pressing the Pellet
The powder-binder mixture is poured into a die and pressed using a hydraulic press. Applying a pressure between 20 and 30 tons compacts the powder into a dense, solid disc with a perfectly flat, non-porous surface ready for analysis.
Understanding the Pitfalls and Trade-offs
While the process is straightforward, errors in preparation can easily invalidate your results. Awareness of these common issues is critical for maintaining data quality.
The Risk of Contamination
The grinding process itself can be a source of contamination. Grinding vessels made of tungsten carbide, for example, can introduce tungsten (W) into your sample. Always choose a grinding material that will not interfere with the elements you are trying to measure.
Inconsistent Grinding and Mixing
Failing to grind the sample finely enough or mix it homogeneously with the binder will reintroduce the very particle size and mineralogical effects you are trying to eliminate. This is a common source of poor repeatability between samples.
Poor Pellet Integrity
Insufficient pressure or an incorrect amount of binder can result in a fragile pellet that cracks or crumbles. Any surface imperfection, including cracks, chips, or even a slightly convex surface, will compromise the quality of the analysis.
Making the Right Choice for Your Goal
The required level of preparation depends entirely on your analytical objective.
- If your primary focus is high-accuracy quantitative analysis: You must adhere strictly to the pressed pellet method, ensuring consistent grinding, precise binder ratios, and controlled pressing conditions for all samples and standards.
- If your primary focus is rapid qualitative screening: Analyzing the material as a loose powder in a sample cup may be sufficient. This is faster but sacrifices the precision needed for true quantitative work.
- If your primary focus is measuring trace elements: Your top priority must be avoiding contamination. This involves carefully selecting binder materials and grinding vessels that are free of the elements you are looking for.
Ultimately, diligent and consistent sample preparation is the foundation upon which all reliable XRF data is built.
Summary Table:
| Step | Key Action | Critical Parameter |
|---|---|---|
| 1. Grinding | Reduce sample to fine powder | Particle size <75 μm |
| 2. Mixing | Combine powder with a binding agent | Homogeneous mixture |
| 3. Pressing | Compress mixture in a hydraulic press | Pressure: 20-30 tons |
Ready to achieve laboratory-grade accuracy with your XRF analysis?
The sample preparation process is the most critical step for reliable data. KINTEK specializes in the high-performance lab equipment and consumables you need for perfect sample prep every time.
We provide the essential tools for success:
- Robust Mills & Grinders for achieving the crucial <75 μm particle size.
- Reliable Hydraulic Presses for creating durable, flat pellets at 20-30 tons.
- High-Purity Binders & Dies to prevent contamination and ensure pellet integrity.
Let's build the foundation for your success. Contact our experts today to discuss your specific application and ensure your sample preparation is optimized for accurate, repeatable results.
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- How are hydraulics used in industry? Unlocking Immense Force for Heavy-Duty Applications
- How does a laboratory hydraulic press contribute to the preparation of green pellets for nanostructured eutectic steel?
- How do I choose a press? A Guide to Matching Your Specific Operational Needs
- What critical process conditions does a laboratory precision hydraulic press provide for fabricating composites?
- What can you do with a hydraulic press? A Versatile Tool for Industrial and Scientific Applications
- How to make a KBr disc? Master the FTIR Pellet Technique for Clear, Accurate Spectra
- What is the limitation of XRF? Understanding the Key Constraints for Accurate Analysis
- What is the problem of a hydraulic press machine? Understanding Operational Trade-offs



















