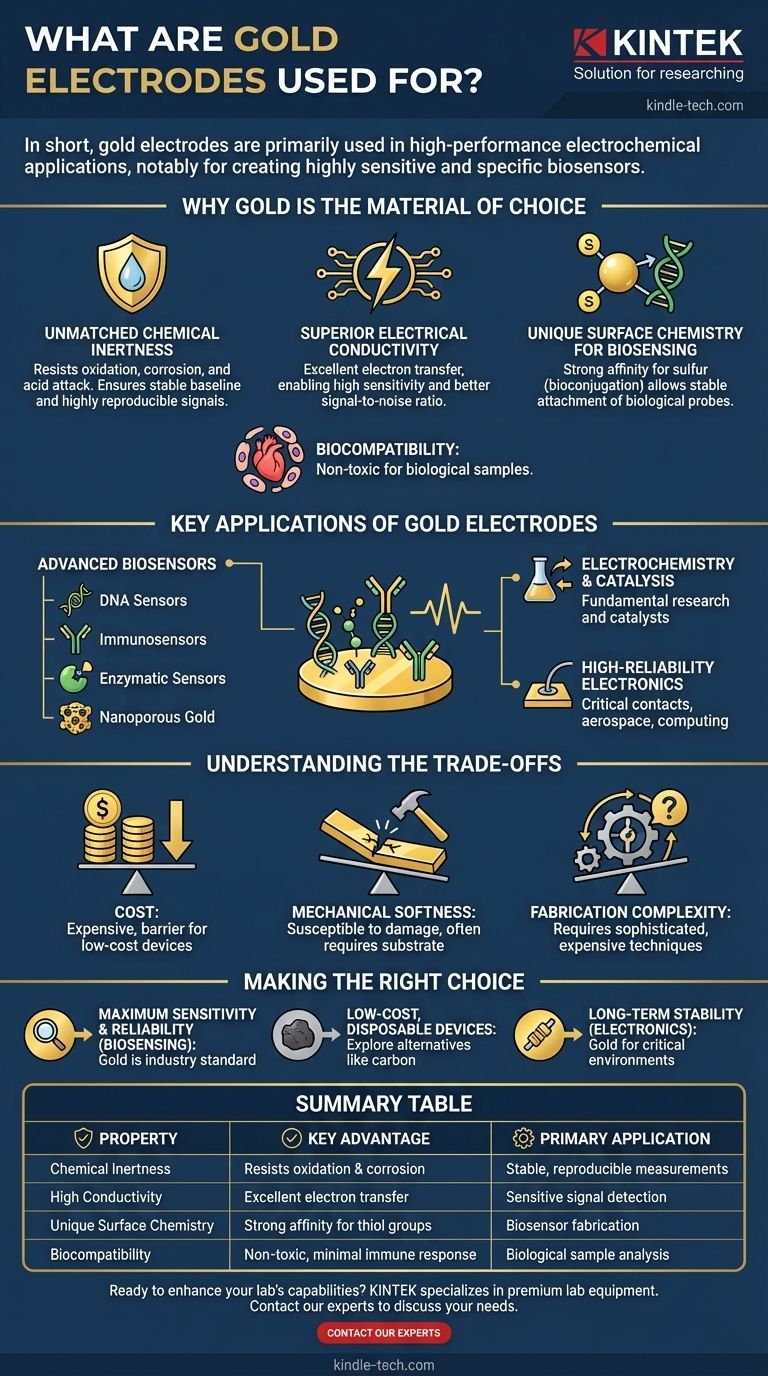
In short, gold electrodes are primarily used in high-performance electrochemical applications, most notably for creating highly sensitive and specific biosensors. Their unique combination of properties makes them the material of choice for detecting specific biological molecules like DNA, proteins, and enzymes, as well as for fundamental electrochemistry research.
The core reason for using gold is not its monetary value, but its unparalleled combination of chemical inertness, high conductivity, and a unique surface chemistry that allows for the stable attachment of biological molecules. This trio of properties is difficult to find in any other material.

Why Gold is the Material of Choice
The selection of gold for electrodes is a deliberate engineering decision rooted in its fundamental physical and chemical properties. These attributes directly address the core challenges of creating a reliable and sensitive electrochemical interface.
Unmatched Chemical Inertness
An electrode's surface must remain stable and unchanged during a measurement. Gold is a noble metal, meaning it is extremely resistant to oxidation, corrosion, and acid attack.
This inertness ensures that the electrode itself does not interfere with the chemical reaction being measured. It guarantees a stable baseline and highly reproducible signals over time, which is critical for scientific accuracy.
Superior Electrical Conductivity
The fundamental job of an electrode is to facilitate the transfer of electrons. Gold is one of the most electrically conductive metals, second only to silver and copper.
This high conductivity ensures that the electrical signal generated by a chemical reaction is transmitted with minimal loss or distortion, leading to higher sensitivity and a better signal-to-noise ratio in measurements.
Unique Surface Chemistry for Biosensing
This is arguably the most important property for biosensor applications. Gold surfaces have a strong, natural affinity for sulfur.
Biological molecules, such as DNA strands or proteins, can be modified with a sulfur-containing group (a thiol). This allows them to form a strong, stable, and highly organized bond directly onto the gold electrode surface. This process, known as bioconjugation, is the key to creating sensors that can selectively detect a single target molecule.
Biocompatibility
Gold is largely non-toxic and does not provoke a significant immune response within biological systems. This makes it an ideal material for sensors that come into contact with biological samples, including blood, saliva, or cell cultures.
Key Applications of Gold Electrodes
The properties of gold make it indispensable in several cutting-edge fields. It enables measurements and devices that would be unreliable or impossible with other materials.
Advanced Biosensors
As mentioned in the reference, this is the most prominent application. The ability to anchor specific biological probes to a gold surface allows for the creation of various sensors:
- DNA Sensors: A single strand of DNA is attached to the gold. If its complementary strand is present in a sample, it binds, creating a detectable electrical signal.
- Immunosensors: Antibodies are attached to the gold to detect the presence of specific antigens (e.g., from a virus), or vice-versa.
- Enzymatic Sensors: An enzyme is attached to the gold. When its target substrate (like glucose) is present, the enzyme catalyzes a reaction that the electrode can measure.
- Nanoporous Gold: Using structured gold, such as nanoporous gold, dramatically increases the surface area available for binding. This significantly amplifies the signal, allowing for the detection of extremely low concentrations of a target molecule.
Electrochemistry and Catalysis
In fundamental research, gold electrodes serve as a clean, well-defined, and inert surface for studying electron transfer reactions. Their predictable behavior makes them an ideal workbench for electrochemists. In some cases, gold nanoparticles also act as highly effective catalysts for specific chemical reactions.
High-Reliability Electronics
While not a sensing application, gold's resistance to oxidation makes it the material of choice for high-quality electrical contacts, wire bonds, and connectors in aerospace, military, and high-end computing hardware where failure is not an option. A copper contact can oxidize over time, increasing resistance and leading to failure; a gold contact will not.
Understanding the Trade-offs
No material is perfect for every situation. Objectivity requires acknowledging gold's limitations.
The Obvious Factor: Cost
Gold is an expensive precious metal. Its high cost is the single biggest barrier to its use in low-cost, disposable, mass-market devices. This is why materials like screen-printed carbon are often used for applications like consumer glucose test strips.
Mechanical Softness
Gold is a very soft and malleable metal. This makes pure gold electrodes susceptible to scratching and physical damage. To overcome this, gold is typically applied as a thin film over a more robust substrate like silicon, glass, or ceramic.
Fabrication Complexity
Creating the highly-ordered or nanostructured gold surfaces needed for advanced sensors requires sophisticated and expensive techniques like physical vapor deposition, electroplating, or dealloying. This adds to the overall cost and complexity of the final device.
Making the Right Choice for Your Application
Your choice of electrode material should be driven entirely by your project's primary goal.
- If your primary focus is maximum sensitivity and reliability in biosensing: Gold is the industry standard due to its unmatched inertness and well-understood surface chemistry for bioconjugation.
- If your primary focus is a low-cost, disposable device for mass production: The high cost of gold is likely prohibitive, and you should investigate alternatives like carbon, platinum, or modified polymers.
- If your primary focus is long-term stability in harsh electronic environments: Gold's resistance to oxidation makes it an excellent choice for critical electrical contacts where failure cannot be tolerated.
Ultimately, selecting gold is a strategic decision to prioritize performance, stability, and reliability where it matters most.
Summary Table:
| Property | Key Advantage | Primary Application |
|---|---|---|
| Chemical Inertness | Resists oxidation & corrosion | Stable, reproducible measurements |
| High Conductivity | Excellent electron transfer | Sensitive signal detection |
| Unique Surface Chemistry | Strong affinity for thiol groups | Biosensor fabrication (DNA, proteins) |
| Biocompatibility | Non-toxic, minimal immune response | Biological sample analysis |
Ready to enhance your lab's capabilities with high-performance gold electrodes? KINTEK specializes in premium lab equipment and consumables, providing reliable solutions for your most sensitive biosensing and electrochemistry research. Our expertise ensures you get the right tools for maximum accuracy and stability. Contact our experts today to discuss your specific needs and discover how KINTEK can support your laboratory's success.
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- How should a gold disc electrode be maintained for long-term use? A Guide to Consistent Performance
- What is the typical role of a gold disc electrode in an electrochemical setup? Your Guide to a Precise Working Electrode
- What are the key precautions for a gold disc electrode? Ensure Accurate Results & Long Lifespan
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data
- What is the proper post-treatment and storage procedure for a gold disc electrode? Ensure Reliable Electrochemical Data



















