Synthesizing nanomaterials is a battle for control. The primary challenges are not just in creating particles at the nanoscale, but in precisely dictating their size, shape, and purity. Traditional methods often involve complex processes and harsh conditions, making it difficult to produce uniform nanoparticles consistently and affordably.
The central issue in nanomaterial synthesis is the immense difficulty of translating precise, laboratory-scale control over particle properties into a process that is scalable, cost-effective, and safe for real-world applications.

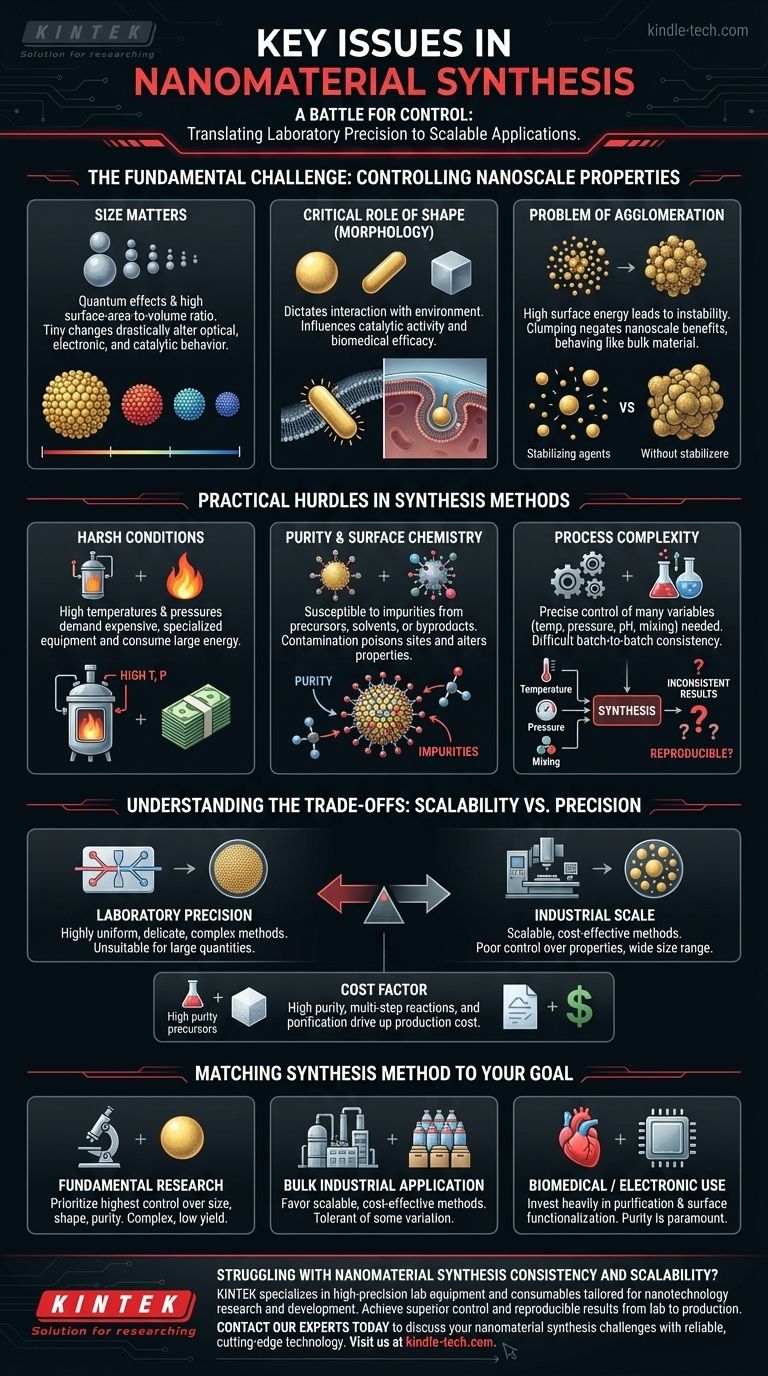
The Fundamental Challenge: Controlling Nanoscale Properties
The unique and powerful properties of nanomaterials are directly tied to their physical dimensions. Any inability to control these dimensions during synthesis directly compromises their final performance.
Why Size Matters
At the nanoscale, properties are no longer constant. They are dictated by quantum effects and an extremely high surface-area-to-volume ratio.
A tiny change in particle diameter—even just a few nanometers—can dramatically alter a material's optical, electronic, or catalytic behavior. For example, the color of gold nanoparticles is entirely dependent on their size.
The Critical Role of Shape (Morphology)
A material's shape determines how it interacts with its environment. A nanosphere, a nanorod, and a nanocube of the same material will have different catalytic activities and optical properties.
In biomedical applications, shape influences how a nanoparticle interacts with cell membranes, making it a critical factor for drug delivery or medical imaging.
The Problem of Agglomeration
Nanoparticles have incredibly high surface energy, which makes them inherently unstable. They have a strong tendency to agglomerate, or clump together, to reach a more stable, lower-energy state.
This clumping negates the benefits of the nanoscale, as a large agglomerate behaves like a bulk material. A significant portion of synthesis is dedicated to preventing this, often through the use of stabilizing agents or "capping" ligands.
Practical Hurdles in Synthesis Methods
Beyond the theoretical challenges of controlling properties, the practical realities of the synthesis process present their own set of significant hurdles.
The Issue of Harsh Conditions
As noted in traditional approaches, many synthesis methods require high temperatures and high pressures.
These conditions demand specialized, expensive equipment, consume large amounts of energy, and are inherently difficult to scale up from a small laboratory reactor to an industrial production vessel.
Ensuring Purity and Surface Chemistry
Nanomaterials are extremely susceptible to impurities. Leftover chemical precursors, solvents, or byproducts from the reaction can adsorb onto the nanoparticle surface.
This contamination can poison catalytic sites, alter electronic properties, or introduce toxicity, making the material useless or dangerous for its intended application.
The Complexity of the Process
Successful synthesis often depends on the precise control of numerous variables: temperature, pressure, pH, reactant concentrations, and mixing rates.
The interplay between these factors is highly complex, making it difficult to achieve batch-to-batch consistency. A process that works perfectly one day may yield a completely different result the next if a single variable is not perfectly replicated.
Understanding the Trade-offs: Scalability vs. Precision
There is often a direct conflict between the ability to produce large quantities of a nanomaterial and the ability to control its properties with high precision.
Laboratory Precision vs. Industrial Scale
Methods that produce highly uniform, "monodisperse" nanoparticles in a lab setting are often delicate and complex, making them unsuitable for producing the kilogram or ton quantities needed for commercial products.
Conversely, methods that are easily scalable, such as mechanical milling (top-down synthesis), often produce particles with a wide range of sizes and shapes, offering poor control over final properties compared to chemical (bottom-up) synthesis.
The Cost Factor
High-purity chemical precursors, complex multi-step reactions, and extensive purification processes all drive up the cost of production.
This economic barrier is a major reason why many promising nanomaterials discovered in academia have not yet transitioned into widespread commercial use.
Matching the Synthesis Method to Your Goal
The "best" synthesis strategy is entirely dependent on your final objective. Understanding your primary goal is the first step toward navigating these challenges.
- If your primary focus is fundamental research: Prioritize methods that offer the highest degree of control over size, shape, and purity, even if they are complex and have low yield.
- If your primary focus is a bulk industrial application: Favor scalable, cost-effective methods and design your product to be tolerant of some variation in nanoparticle properties.
- If your primary focus is biomedical or electronic use: Invest heavily in purification and surface functionalization steps, as purity and surface chemistry are paramount.
Mastering these synthesis challenges is the key that unlocks the transformative potential of nanotechnology.
Summary Table:
| Challenge | Key Issue | Impact on Final Product |
|---|---|---|
| Size Control | Quantum effects and surface-area-to-volume ratio | Drastic changes in optical, electronic, and catalytic properties |
| Shape Control | Morphology dictates interaction with environment | Affects catalytic activity and biomedical application efficacy |
| Purity & Surface Chemistry | Contamination from precursors or solvents | Can poison catalytic sites or introduce toxicity |
| Scalability | Trade-off between lab precision and industrial production | High cost and batch inconsistency hinder commercial use |
Struggling with nanomaterial synthesis consistency and scalability? KINTEK specializes in providing high-precision lab equipment and consumables tailored for nanotechnology research and development. Our solutions help you achieve superior control over particle size, shape, and purity, ensuring reproducible results from lab to production. Contact our experts today to discuss how we can support your nanomaterial synthesis challenges with reliable, cutting-edge technology.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Anti-Cracking Press Mold for Lab Use
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- What is the role of standard sieves in gold scrap leaching kinetic studies? Ensure Precision in Particle Classification
- What is the primary purpose of using standard sieves? Master Particle Uniformity for High-Quality Catalyst Preparation
- What are the specifications for test sieves? A Guide to ASTM & ISO Standards for Accurate Particle Analysis
- Why is sieve analysis important? Ensure Consistent Quality and Performance of Your Materials
- What is the primary function of a mechanical sieve shaker for biomass analysis? Optimize Particle Size Distribution



















