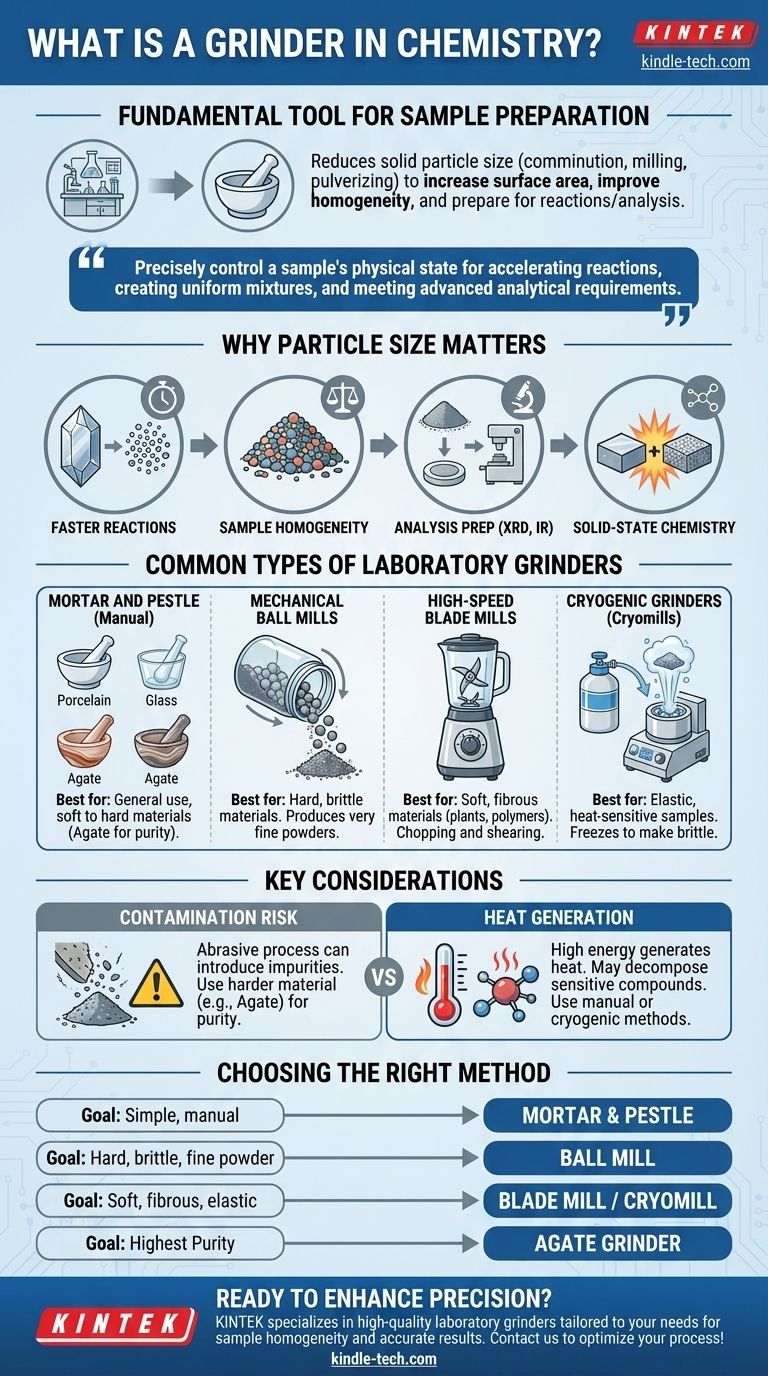
In chemistry, a grinder is a fundamental tool for sample preparation used to reduce the particle size of a solid substance. This process, also known as comminution, milling, or pulverizing, is not merely about making things smaller. It is a critical step that increases a sample's surface area, improves its homogeneity, and prepares it for subsequent chemical reactions or analytical procedures.
The central purpose of a grinder isn't just to break down materials; it's to precisely control a sample's physical state. This control is essential for accelerating reactions, creating uniform mixtures, and meeting the strict requirements of advanced analytical techniques.

Why Particle Size is Critical in Chemistry
Reducing a solid to a fine, uniform powder has profound effects on its chemical and physical behavior. The choice of grinding method is often the first step in determining the success of an experiment.
Increasing Surface Area for Faster Reactions
The rate of a chemical reaction involving a solid is directly proportional to its surface area. By breaking a large crystal into millions of smaller particles, you dramatically increase the number of atoms exposed and available to react.
Think of dissolving a sugar cube versus granulated sugar in water. The granulated sugar dissolves much faster because its total surface area is vastly larger.
Ensuring Sample Homogeneity
Most solids are not perfectly uniform. To get a representative sample for analysis, you must grind a larger portion into a fine powder and mix it thoroughly.
This ensures that the small amount you weigh for analysis accurately reflects the composition of the bulk material, preventing inaccurate and non-reproducible results.
Preparing Samples for Analysis
Many modern analytical techniques have strict sample requirements. Techniques like X-ray Diffraction (XRD) require a fine, randomly oriented powder to produce a clear pattern.
Similarly, methods like infrared (IR) spectroscopy often involve mixing the sample with a powder like potassium bromide (KBr) and pressing it into a pellet, a process that requires uniform, small particles.
Enabling Solid-State Chemistry
A specialized field called mechanochemistry uses mechanical force, often from high-energy ball milling, to initiate chemical reactions directly between solids.
In these cases, the grinder is not just for preparation; it is the reaction vessel itself, with the grinding action providing the energy needed to break and form chemical bonds.
Common Types of Laboratory Grinders
The right tool depends entirely on the sample's properties—its hardness, brittleness, and thermal stability.
The Manual Mortar and Pestle
This is the most classic and simple grinding tool. The choice of material is critical.
- Porcelain: Good for general-purpose grinding of softer materials.
- Glass: Suitable for chemicals that may stain porcelain.
- Agate: Extremely hard and non-porous, used for grinding hard solids when minimizing contamination is essential.
Mechanical Ball Mills
A ball mill uses a rotating jar containing the sample and grinding media (e.g., ceramic or steel balls). As the jar rotates, the balls cascade and tumble, crushing the material through impact and attrition.
This method is highly effective for producing very fine powders from hard and brittle materials.
High-Speed Blade Mills
These are essentially specialized, high-power blenders. A motor rotates sharp blades at very high speeds, chopping and shearing the sample.
This approach works best for soft, non-brittle, or fibrous materials like plant tissue, polymers, or food products.
Cryogenic Grinders (Cryomills)
Some samples, like plastics, rubber, or certain biological tissues, are too soft or elastic to grind at room temperature. They simply deform or melt from the heat of friction.
A cryogenic grinder first freezes the sample in liquid nitrogen, making it extremely brittle. The frozen sample is then easily shattered into a fine powder.
Understanding the Trade-offs and Considerations
Choosing a grinding method is a balance of efficiency, purity, and sample integrity.
Contamination Risk
Grinding is an abrasive process. The grinder's surface (e.g., the mortar or the balls in a mill) can wear down and introduce impurities into your sample. This is why an agate mortar and pestle is used for high-purity applications—agate is harder than most chemical samples.
Heat Generation
High-energy grinding generates significant heat. This can be enough to decompose a thermally sensitive compound or alter its crystalline structure. For these materials, manual grinding or cryogenic milling are the only viable options.
Material Properties Matter
You cannot use one grinder for all tasks. Using a blade mill on a hard mineral will damage the blades and achieve nothing. Using a ball mill on a soft polymer will likely just melt the sample onto the walls of the jar. The sample's hardness and thermal stability dictate the proper technique.
Choosing the Right Grinding Method
Your experimental goal determines the correct tool for the job.
- If your primary focus is simple, manual size reduction for non-critical applications: A standard porcelain mortar and pestle is the most cost-effective choice.
- If you need to grind hard, brittle materials into a very fine, uniform powder: A mechanical ball mill provides the necessary energy and consistency.
- If you are working with soft, fibrous, or elastic samples like polymers or plant tissue: A high-speed blade mill or a cryogenic grinder is necessary to overcome their ductility.
- If sample purity is your absolute highest priority: Choose a grinder made of a material harder than your sample, such as agate, to minimize contamination.
Ultimately, selecting the correct grinder is the first critical step in ensuring the reliability and success of your subsequent chemical analysis or reaction.
Summary Table:
| Grinder Type | Best For | Key Characteristic |
|---|---|---|
| Mortar & Pestle | Soft materials, general use | Manual, simple, various materials (porcelain, agate) |
| Ball Mill | Hard, brittle materials | Produces fine powders via impact/attrition |
| Blade Mill | Soft, fibrous materials (e.g., plants) | High-speed chopping and shearing |
| Cryogenic Grinder | Elastic, heat-sensitive samples | Uses liquid nitrogen to make samples brittle |
Ready to enhance your lab's precision and efficiency?
Choosing the right grinder is the critical first step for reliable sample preparation. KINTEK specializes in high-quality laboratory equipment and consumables, including a full range of grinders and mills tailored to your specific chemical analysis needs. Our experts can help you select the perfect tool to ensure sample homogeneity, prevent contamination, and achieve accurate results.
Contact KINTEK today to discuss your lab's grinding requirements and let us help you optimize your sample preparation process!
Visual Guide
Related Products
- Laboratory Hybrid Tissue Grinding Mill
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Lab Sterile Slapping Type Homogenizer for Tissue Mashing and Dispersing
- High Energy Planetary Ball Mill Machine for Laboratory Horizontal Tank Type
- Low-Temperature Water-Cooled Touchscreen Vibratory Ultrafine Pulverizer
People Also Ask
- How full should a ball mill be? Achieve Peak Grinding Efficiency with the 50% Rule
- How does particle size affect XRF? Achieve Accurate and Repeatable Elemental Analysis
- What are the five methods of synthesis of nanoparticles? A Guide to Top-Down & Bottom-Up Approaches
- What are the different types of grinding mills? Match the Mechanism to Your Material for Optimal Size Reduction
- How many balls are needed for a ball mill? Optimize grinding with the right charge volume.



















