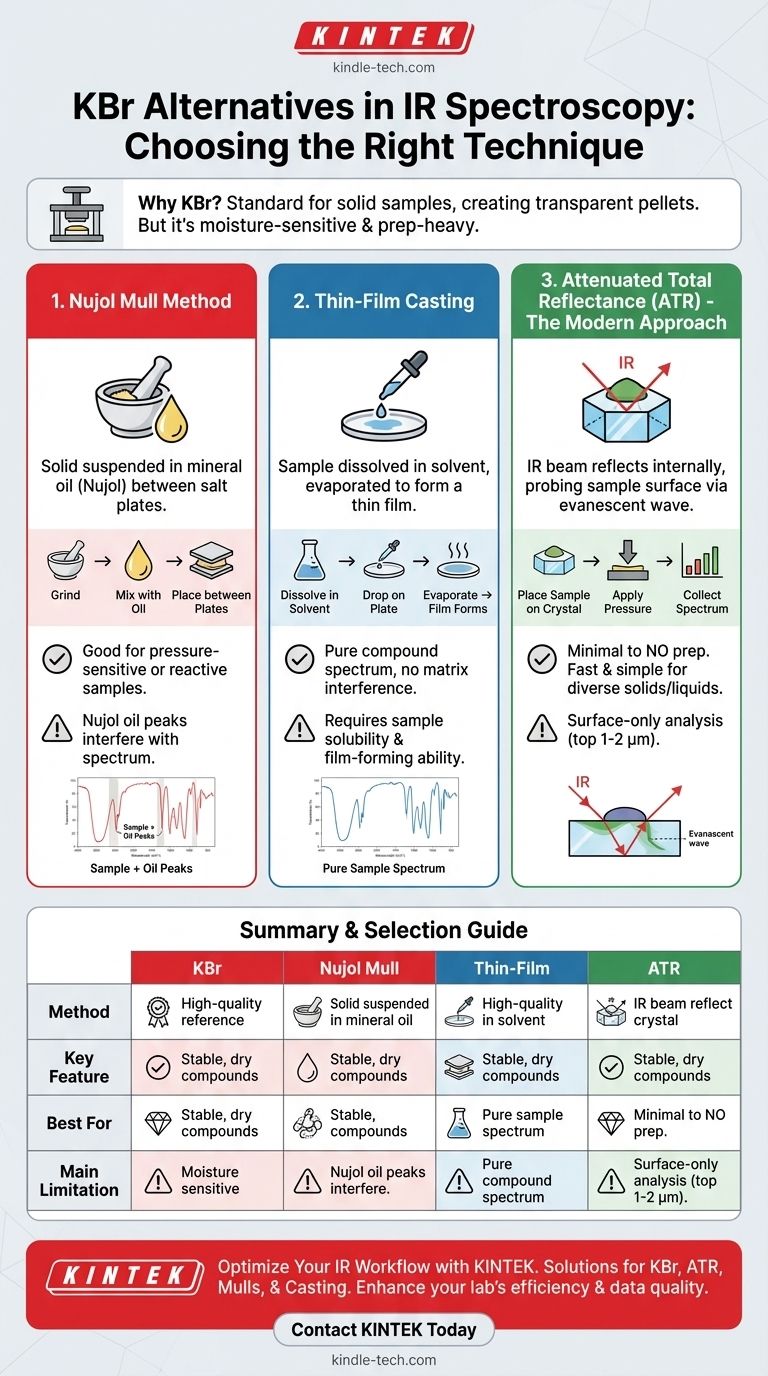
While KBr is the most common material for preparing solid samples, several powerful alternatives exist for IR spectroscopy, primarily revolving around different sample preparation techniques. The most common alternatives are the Nujol mull technique, thin-film casting from a solvent, and the modern, increasingly prevalent method of Attenuated Total Reflectance (ATR). The best choice is dictated by your sample's physical properties, its reactivity, and the desired speed of analysis.
The core challenge in solid-state IR spectroscopy is not just finding a substitute material for KBr, but selecting the right technique to make your sample transparent to the IR beam. The most practical and versatile alternative for most modern labs is ATR, as it requires virtually no sample preparation.

Why KBr is the Standard
To understand the alternatives, it's essential to first understand why potassium bromide (KBr) became the benchmark for solid sample analysis. The entire method is built on a few key properties.
The Principle of IR Transparency
Alkali halides, the family of salts that includes KBr, do not absorb light in the mid-infrared region. This makes them ideal "windows" through which to pass an IR beam, ensuring the resulting spectrum is purely from the sample, not the matrix.
Creating a Solid Solution
Under high pressure, KBr powder becomes plastic and flows, forming a uniform, transparent sheet or "pellet." When a finely ground sample is mixed in, it becomes trapped and evenly dispersed within this transparent KBr matrix, allowing the IR beam to pass through it effectively.
The Need for Purity and Dryness
The KBr method requires that both the KBr and the sample be exceptionally dry. Water (moisture) has very strong IR absorption bands that can easily obscure important features in a sample's spectrum. This sensitivity to moisture is one of the primary drivers for seeking alternative methods.
Key Alternatives to the KBr Pellet
When the KBr pellet method is unsuitable—due to moisture sensitivity, sample reactivity, or time constraints—analysts turn to other established techniques.
The Nujol Mull Method
A mull is a thick paste created by grinding a solid sample with a mulling agent. The sample is not dissolved, but suspended.
How It Works A small amount of the solid sample is ground to a fine powder and then mixed with a drop or two of a mulling agent, most commonly mineral oil (Nujol). This paste is then spread between two IR-transparent salt plates (often made of KBr or NaCl) to be placed in the spectrometer. The oil helps reduce scattering of the IR beam.
Inherent Spectral Interference The major drawback is that the mulling agent itself has an IR spectrum. Nujol is a hydrocarbon and has prominent C-H stretching and bending bands. Analysts must be aware of these peaks and disregard them when interpreting the sample's spectrum.
Thin-Film Casting
This method is ideal for samples that are soluble in a volatile solvent, particularly polymers.
The Preparation Process The sample is dissolved in a suitable solvent (like acetone or dichloromethane). A drop of this solution is placed on an IR-transparent salt plate, and the solvent is allowed to evaporate completely. This leaves behind a thin, uniform film of the pure sample, ready for analysis.
Advantages and Limitations The primary advantage is that the resulting spectrum is of the pure compound, with no interference from a matrix like KBr or a mulling agent. The main limitation is that the sample must be soluble and able to form a coherent film upon drying.
The Modern Approach: Attenuated Total Reflectance (ATR)
For most modern laboratories, ATR has become the go-to alternative for its simplicity and speed, often replacing KBr and mull techniques for routine analysis.
How ATR Works
ATR works on a different principle. The IR beam is directed into a special crystal (often diamond, germanium, or zinc selenide) with a high refractive index. The sample is pressed firmly against the surface of this crystal. The beam reflects internally within the crystal, but a small portion of the energy, called an evanescent wave, penetrates a few micrometers into the sample. Where the sample absorbs energy, the beam is attenuated (weakened), generating the spectrum.
The Advantage of Minimal Preparation
This is the key benefit of ATR. Solid powders, films, and even liquids can be analyzed directly with almost no preparation. You simply place the sample on the crystal, apply pressure to ensure good contact, and collect the spectrum. This eliminates the need for grinding, pressing pellets, or dealing with moisture.
Understanding the Trade-offs
No single method is perfect for every situation. Choosing an alternative means accepting a different set of compromises.
KBr Pellets: Potential for Error
The KBr method is often considered the "gold standard" for reference spectra but is prone to error. Moisture contamination is the most common problem. Furthermore, the high pressure used to form the pellet can sometimes alter the crystalline structure of a sample, leading to a different spectrum than the native material.
Nujol Mulls: Inevitable Contamination
With a mull, you must accept that the spectrum will always contain interfering peaks from the mulling agent. This can be problematic if your sample's key absorption bands overlap with those of the oil.
ATR: Surface-Only Analysis
The biggest limitation of ATR is that it is a surface technique. The evanescent wave only probes the top 1-2 micrometers of the sample. If the surface of your sample is not representative of the bulk (due to oxidation, for example), your spectrum will be misleading. Achieving good, consistent contact between the sample and the crystal is also critical for reproducibility.
Making the Right Choice for Your Sample
Your choice of sample preparation should be dictated by your sample's physical properties and your analytical goals.
- If your primary focus is a high-quality reference spectrum of a stable compound: A carefully prepared KBr pellet is often the best choice, assuming you can eliminate moisture.
- If your primary focus is rapid, routine analysis of diverse solid materials: ATR is unmatched for its speed, ease of use, and minimal sample preparation.
- If your sample is sensitive to pressure or known to react with KBr: A Nujol mull is a classic and effective alternative that avoids these issues.
- If your sample is a soluble polymer or film-forming material: Thin-film casting provides a pure spectrum of your material without any matrix interference.
Ultimately, understanding these methods empowers you to select the optimal path for generating a clean and accurate infrared spectrum.
Summary Table:
| Method | Key Feature | Best For | Main Limitation |
|---|---|---|---|
| KBr Pellet | High-quality reference spectrum | Stable, dry compounds | Sensitive to moisture |
| ATR | Minimal to no sample preparation | Rapid, routine analysis | Surface-only analysis |
| Nujol Mull | Avoids high pressure | Pressure-sensitive or reactive samples | Spectral interference from oil |
| Thin-Film Casting | Pure compound spectrum | Soluble, film-forming materials | Requires specific solubility |
Optimize Your IR Spectroscopy Workflow with KINTEK
Choosing the right sample preparation method is critical for obtaining accurate and reliable IR spectra. Whether your lab prioritizes the reference quality of KBr pellets, the speed and simplicity of ATR, or the specific advantages of mulls and casting, having the right equipment is key.
KINTEK specializes in providing high-quality lab equipment and consumables to support all your IR spectroscopy needs. We can help you select the perfect presses for KBr pellets, durable ATR accessories, or the necessary materials for mull and thin-film techniques.
Let our experts help you enhance your lab's efficiency and data quality.
Contact KINTEK today to discuss your specific requirements and discover how our solutions can benefit your research and analysis.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for Sampling Filters
- Custom PTFE Teflon Parts Manufacturer for Air Valve Applications
- Optical Window Glass Substrate Wafer Barium Fluoride BaF2 Substrate Window
- Laboratory High Throughput Tissue Grinding Mill Grinder
- Automatic Laboratory Heat Press Machine
People Also Ask
- What is the role of a laboratory hydraulic press in evaluating concrete? Test Eggshell Additive Performance
- What is the function of a laboratory hydraulic press in nanocellulose prep? Unlock Ultra-High Strength Materials
- What can KBr be used as? The Essential Matrix for Accurate FTIR Spectroscopy
- What are hydraulic presses used for? Powering Industries with Immense, Controlled Force
- What happens when hydraulics overheat? Prevent Catastrophic System Failure and Costly Downtime
- What is the difference between brake press and punch press? Choosing the Right Metal Fabrication Tool
- How do laboratory hydraulic presses and specialized fixtures ensure the accuracy of electrochemical testing? (Expert Guide)
- Are hydraulic presses powered by water? Discover the critical role of hydraulic oil.















