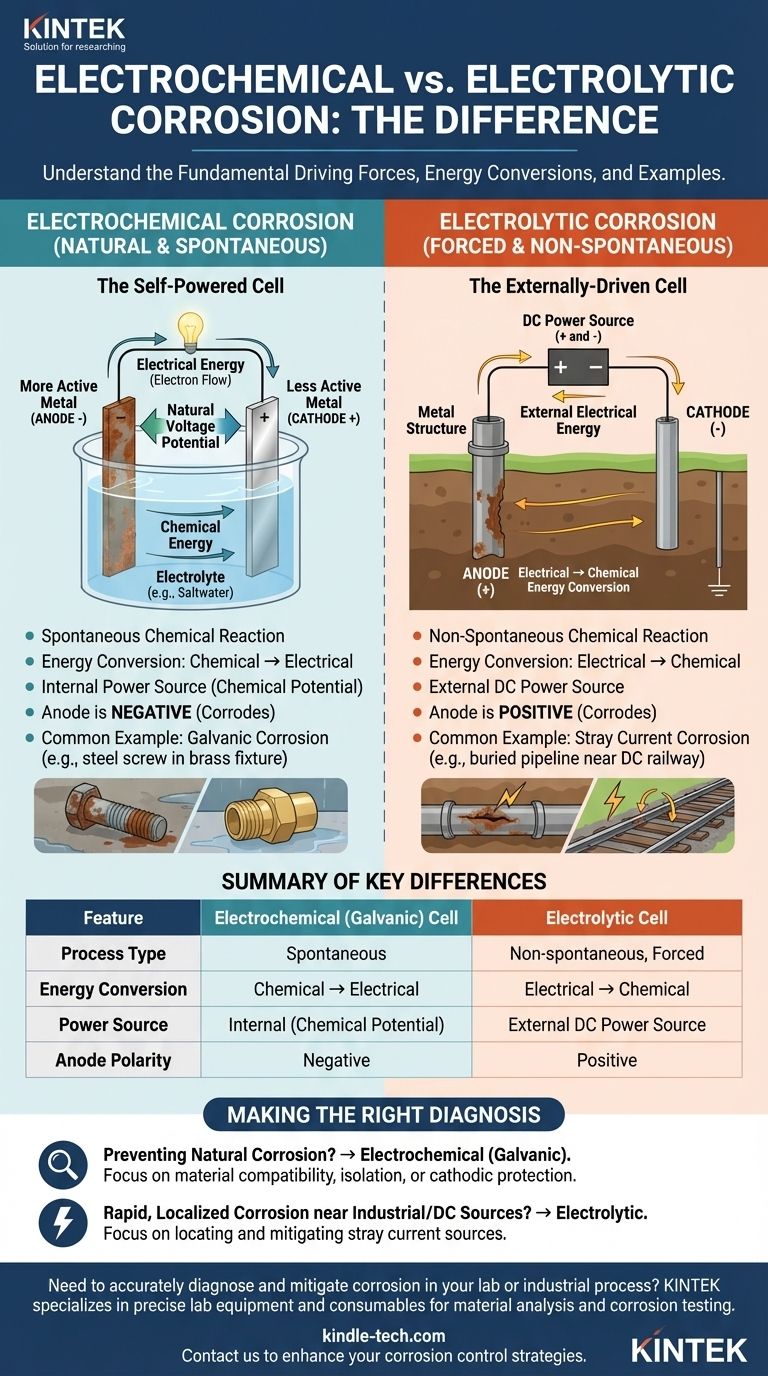
At a fundamental level, the difference between an electrolytic and an electrochemical corrosion cell lies in their energy conversion and driving force. An electrochemical cell is a spontaneous process that converts stored chemical energy into electrical energy, causing natural corrosion. In contrast, an electrolytic cell is a non-spontaneous process that uses external electrical energy to force a chemical reaction, causing induced corrosion.
The core distinction is spontaneity. Electrochemical corrosion happens naturally on its own, like a battery discharging. Electrolytic corrosion is forced upon a material by an external power source, like stray DC current from a rail system.

The Electrochemical Cell: Corrosion's Natural State
An electrochemical cell, often called a galvanic cell, is the mechanism behind the most common forms of corrosion. It is a self-contained, naturally occurring process.
A Spontaneous Chemical Reaction
This type of cell forms when two different metals are in electrical contact in the presence of an electrolyte (like saltwater). A natural voltage potential exists between them.
The more chemically active metal becomes the anode (negative electrode) and corrodes, releasing electrons. The less active metal becomes the cathode (positive electrode) and accepts these electrons.
Energy Conversion: Chemical to Electrical
The driving force is the release of stored chemical energy within the more active metal. This chemical energy is converted directly into electrical energy in the form of electron flow from the anode to the cathode. This process requires no external power.
A classic example is galvanic corrosion, where a steel screw (anode) rusts rapidly when connected to a brass fixture (cathode) in a damp environment.
The Electrolytic Cell: Corrosion by Force
An electrolytic cell drives a chemical reaction that would not normally occur. It does so by applying an external source of electrical power.
A Non-Spontaneous Chemical Reaction
This process overcomes the natural tendencies of the materials involved. The external power source forces a metal to become an anode and corrode, even if it would be stable otherwise.
Corrosion occurs where this externally-supplied direct current (DC) leaves the metal structure to enter an electrolyte.
Energy Conversion: Electrical to Chemical
Here, electrical energy from an outside source is converted into chemical energy, which manifests as the corrosion reaction. The cell consumes power to operate.
A common real-world scenario is stray current corrosion. A buried pipeline running near a DC-powered railway can pick up leaking current, forcing the section of pipe where the current exits back to the soil to corrode at an accelerated rate.
Understanding the Key Differences
While both processes involve anodes, cathodes, and an electrolyte, their fundamental characteristics are opposite. Recognizing these differences is critical for proper diagnosis and mitigation.
Driving Force and Power Source
The most important distinction is the driving force. An electrochemical cell is self-powered by the chemical potential difference between materials. An electrolytic cell is externally powered by an outside DC source.
Polarity of the Electrodes
The polarity of the anode and cathode is reversed between the two cells, a frequent point of confusion.
- In an electrochemical (galvanic) cell, the anode (where corrosion occurs) is negative, and the cathode is positive.
- In an electrolytic cell, the external power source makes the anode (where corrosion occurs) positive, and the cathode is negative.
Practical Implications
Misidentifying the type of corrosion cell leads to incorrect solutions. For example, changing material pairs might solve a galvanic corrosion problem but will do nothing to stop stray current corrosion.
Making the Right Diagnosis
Understanding the underlying mechanism is the first step toward effective corrosion control. Your diagnostic approach should be guided by the suspected cell type.
- If your primary focus is preventing natural corrosion: You are likely dealing with an electrochemical (galvanic) cell. Your solution involves selecting compatible materials, electrically isolating them, or applying cathodic protection.
- If you are investigating rapid, localized corrosion near industrial equipment or DC transit: You are almost certainly dealing with an electrolytic cell. Your priority must be to locate and mitigate the source of the external stray current.
Ultimately, knowing whether the corrosion is happening naturally or being forced by an external influence dictates your entire prevention and control strategy.
Summary Table:
| Feature | Electrochemical (Galvanic) Cell | Electrolytic Cell |
|---|---|---|
| Process Type | Spontaneous | Non-spontaneous, Forced |
| Energy Conversion | Chemical → Electrical | Electrical → Chemical |
| Power Source | Internal (Chemical Potential) | External DC Power Source |
| Anode Polarity | Negative | Positive |
| Common Example | Galvanic Corrosion (e.g., steel/brass) | Stray Current Corrosion (e.g., from railways) |
Need to accurately diagnose and mitigate corrosion in your lab or industrial process?
Understanding the exact type of corrosion cell is the first step to an effective solution. The experts at KINTEK specialize in providing the precise lab equipment and consumables needed for material analysis and corrosion testing. Whether you're investigating galvanic reactions or stray current effects, we have the tools to support your research and ensure material integrity.
Contact us today via our contact form to discuss your specific laboratory needs and discover how KINTEK's solutions can enhance your corrosion control strategies.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Flat Corrosion Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- How does a three-electrode electrolytic cell function? Precision Testing for 8620 Steel in Corrosive Environments
- How does an electrochemical cell system ensure measurement precision during DL-EPR? | Expert Testing Guide
- What are the advantages of a flat electrochemical cell for corrosion? Achieve Precise Pitting & Crevice Analysis
- How is a three-electrode electrochemical electrolytic cell utilized to evaluate Zr-Nb alloy corrosion resistance?
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing



















