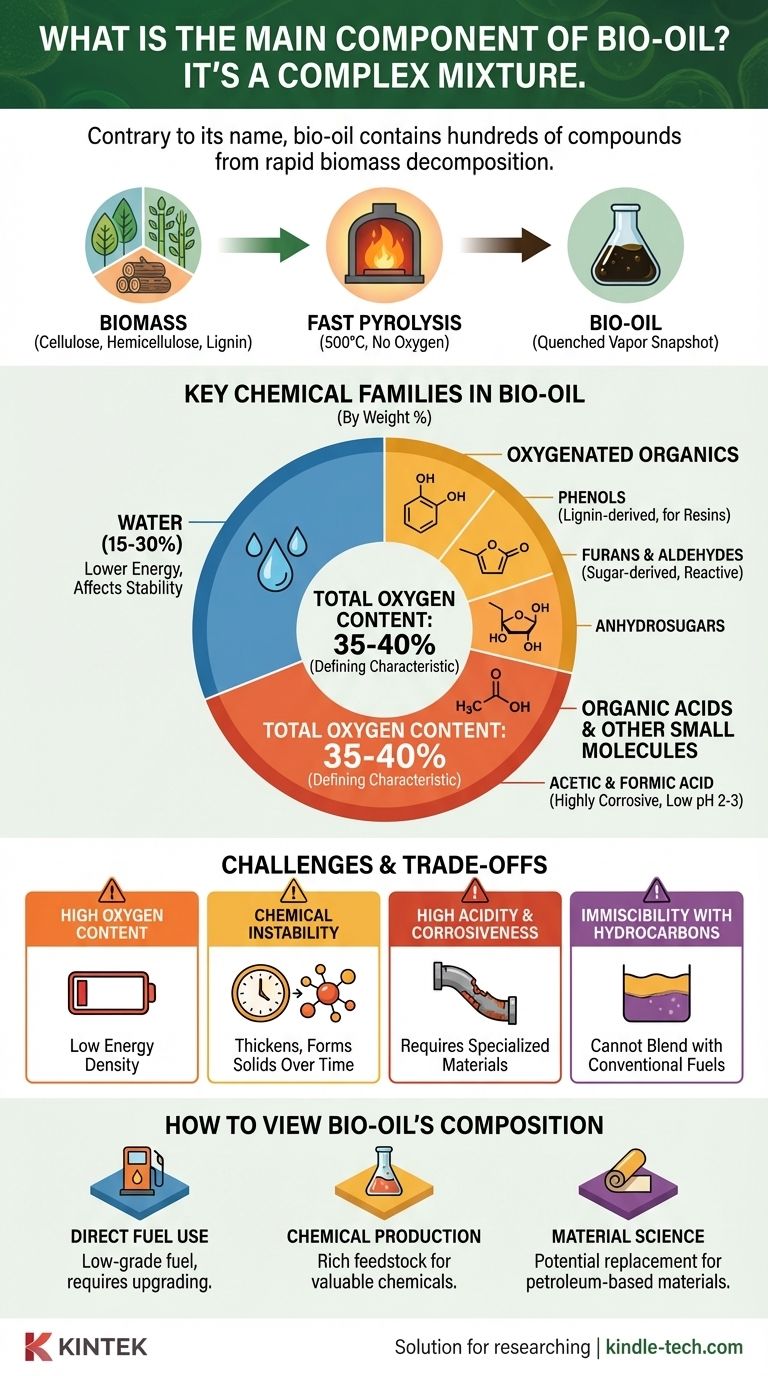
Contrary to what its name suggests, bio-oil does not have a single main component. It is an extremely complex liquid mixture, often called pyrolysis oil, containing hundreds of different oxygenated organic compounds derived from the rapid thermal decomposition of biomass. The most significant components by weight are typically water and a diverse array of oxygenates, including phenols, furans, aldehydes, ketones, and organic acids.
The defining characteristic of bio-oil isn't a single dominant chemical, but its inherent complexity and high oxygen content. This makes it fundamentally different from petroleum crude oil and presents both unique opportunities as a chemical feedstock and significant challenges as a direct fuel.

Why is Bio-Oil So Complex?
Understanding the composition of bio-oil requires looking at its source material and the process used to create it. It is not a naturally occurring substance but the direct product of a rapid, controlled decomposition.
The Source: Deconstructing Biomass
Biomass, such as wood or agricultural waste, is primarily composed of three natural polymers: cellulose, hemicellulose, and lignin. Each of these complex structures breaks down differently, contributing a unique set of chemicals to the final mixture.
The Process: Fast Pyrolysis
Bio-oil is produced through fast pyrolysis, a process that involves heating biomass to around 500°C in the complete absence of oxygen. This intense heat shatters the large biopolymers into a hot vapor of smaller, highly reactive molecules.
The Result: A "Snapshot" of Decomposition
This vapor is then rapidly cooled, or "quenched," which effectively freezes the chemical reactions in place. This traps hundreds of intermediate decomposition products in a liquid state, creating the dark, viscous fluid known as bio-oil.
Key Chemical Families in Bio-Oil
Rather than thinking of a single main component, it is more accurate to categorize the contents of bio-oil into several major chemical families.
Water: The "Hidden" Component
Bio-oil contains a significant amount of water, typically 15-30% by weight. This water comes from the initial moisture in the biomass and from dehydration reactions that occur during pyrolysis. It significantly lowers the oil's energy content and affects its stability.
Lignin-Derived Phenols
The complex, aromatic structure of lignin breaks down to form a variety of phenolic compounds (phenols, guaiacols, and syringols). These are valuable as potential replacements for petroleum-derived phenols in resins and adhesives.
Cellulose and Hemicellulose Derivatives
The breakdown of these sugar-based polymers yields a wide range of oxygenated compounds. Key products include anhydrosugars (like levoglucosan), furans, and various aldehydes and ketones. These molecules are highly reactive.
Acids and Other Small Molecules
Fast pyrolysis also generates small organic acids, primarily acetic acid and formic acid. The presence of these acids makes bio-oil highly corrosive, with a very low pH typically between 2 and 3.
Understanding the Trade-offs and Challenges
The unique chemical makeup of bio-oil is the source of its greatest potential and its most significant problems.
High Oxygen Content
Bio-oil has a very high oxygen content, often 35-40% by weight, compared to less than 1% for conventional crude oil. This oxygen is bound within the molecules and drastically reduces the oil's heating value, or energy density.
Chemical Instability
The presence of highly reactive aldehydes, ketones, and other compounds means bio-oil is unstable. Over time, these molecules can react with each other (polymerize), causing the oil to thicken, form solids, and separate into different phases, which complicates storage and transport.
High Acidity and Corrosiveness
The organic acids in bio-oil make it highly corrosive to common construction materials like carbon steel. This necessitates the use of more expensive, specialized stainless steels and alloys for pipes, tanks, and processing equipment.
Immiscibility with Hydrocarbons
Due to its high concentration of polar, oxygenated molecules, bio-oil does not mix with non-polar hydrocarbon fuels like diesel or gasoline. This prevents simple blending and requires extensive chemical upgrading before it can be used in conventional refineries or engines.
How to View Bio-Oil's Composition
Your interpretation of bio-oil's complex composition depends entirely on your intended application.
- If your primary focus is on direct fuel use: Recognize that its high water and oxygen content make it a low-grade fuel that requires significant upgrading (like hydrodeoxygenation) to be compatible with conventional engines and infrastructure.
- If your primary focus is on chemical production: View the complex mixture not as a flaw, but as a rich feedstock for extracting valuable platform chemicals like phenols, furans, and anhydrosugars.
- If your primary focus is on material science: Consider the phenolic compounds as a potential bio-based replacement for phenol in producing resins, adhesives, and foams.
Ultimately, understanding bio-oil means shifting perspective from seeking a single "main component" to strategically managing its complex and reactive chemical nature.
Summary Table:
| Component/Family | Typical Weight % | Key Characteristics |
|---|---|---|
| Water | 15-30% | Lowers energy density, affects stability |
| Oxygenated Organics (Phenols, Furans, Aldehydes, etc.) | Varies | High reactivity, potential chemical feedstock |
| Organic Acids (Acetic, Formic) | Varies | Causes low pH (2-3), highly corrosive |
| Total Oxygen Content | 35-40% | Fundamental difference from petroleum crude oil |
Need to process or analyze complex materials like bio-oil? KINTEK specializes in high-performance lab equipment and consumables for pyrolysis, chemical analysis, and material testing. Our ovens, reactors, and analytical tools are designed to handle corrosive and reactive substances, ensuring accurate and reliable results for your biomass and energy research. Contact us today to find the right solution for your laboratory challenges! Get in touch
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Assemble Square Lab Press Mold for Laboratory Applications
- Special Heat Press Mold for Lab Use
- Square Lab Press Mold for Laboratory Applications
- Assemble Lab Cylindrical Press Mold
People Also Ask
- How are PTFE gaskets utilized for POEGMA electrolyte conductivity? Ensure Precision in Electrochemical Measurements
- What is the function of PTFE reaction kettle bodies in micro-CSTR systems? Enhance Chemical Stability & Flow
- What is the difference between PPF and coating? Armor vs. Slick Shell for Your Car
- Why are PTFE laboratory consumables required when testing stainless steel against organic acids? Ensure Data Integrity
- What are the advantages of using PTFE molds for epoxy resin flame retardant samples? Ensure High-Purity Material Testing




