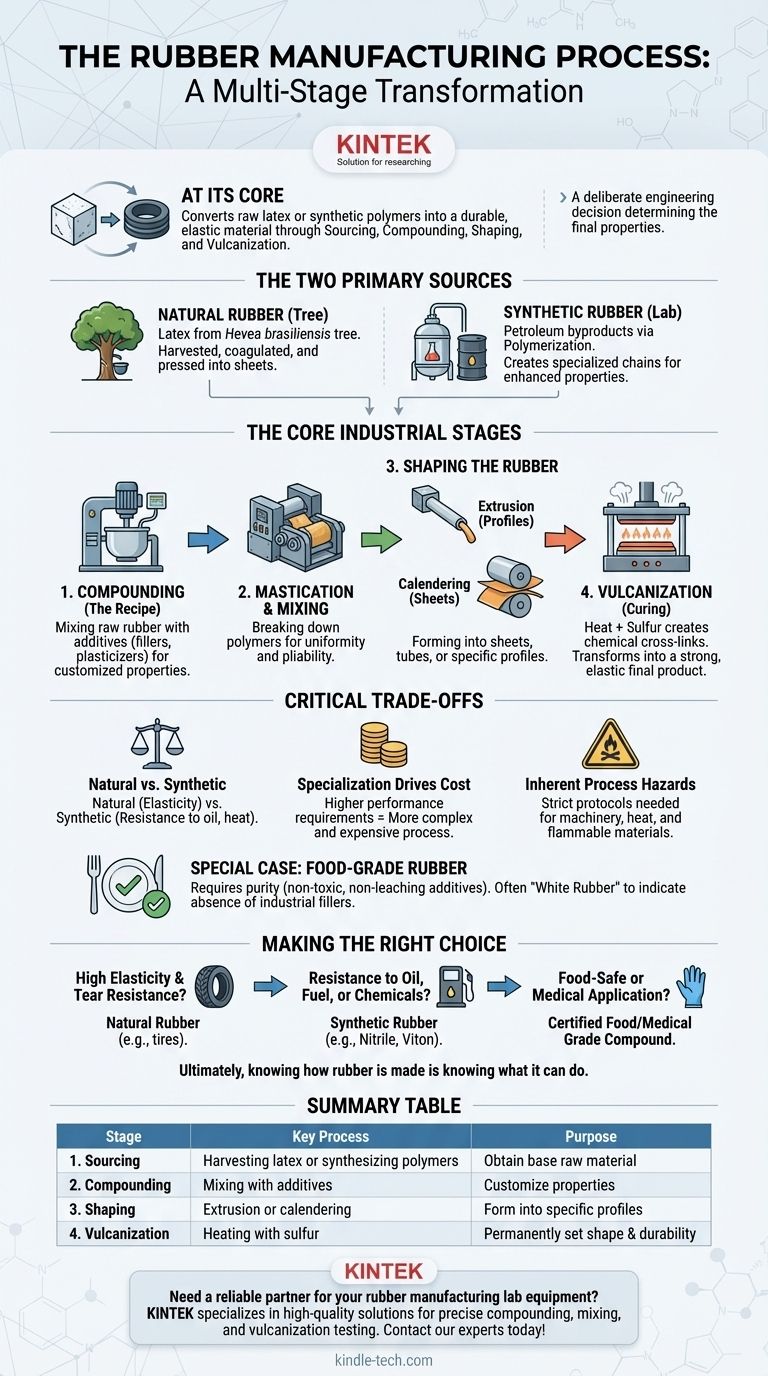
At its core, the rubber manufacturing process is a multi-stage transformation that converts raw latex or synthetic polymers into a durable, elastic material. The primary stages involve sourcing the raw material, mixing it with additives in a process called compounding, shaping it into a desired form, and then curing it with heat in a step known as vulcanization.
The specific manufacturing path chosen is not arbitrary; it is a deliberate engineering decision. Each step, from the choice of raw material to the type of additives used, directly determines the final rubber's strength, flexibility, chemical resistance, and ultimate suitability for its intended application.

The Two Primary Sources of Rubber
The journey begins with one of two distinct starting materials: natural latex from a tree or synthetic polymers created in a lab. This initial choice is the first and most critical factor influencing the final product's properties.
Sourcing Natural Rubber
Natural rubber originates as a milky white sap called latex, which is harvested from the Hevea brasiliensis tree.
Skilled workers, known as tappers, make precise incisions in the tree's bark to collect the sap. This raw latex is then filtered, combined with a mild acid to help it solidify (coagulate), and pressed into large slabs or sheets for transport to a processing factory.
Creating Synthetic Rubber
Synthetic rubbers, such as polyurethane or nitrile, are not grown but are manufactured from petroleum-based byproducts.
Through chemical processes like polymerization, simple molecules (monomers) are linked together into long chains (polymers) that mimic or enhance the properties of natural rubber. This allows for the creation of specialized rubbers with properties unattainable with natural latex, such as extreme temperature or oil resistance.
The Core Industrial Manufacturing Stages
Once the raw rubber (natural or synthetic) arrives at the factory, it undergoes a series of intensive mechanical and chemical processes.
Step 1: Compounding (The Recipe)
This is arguably the most critical stage for customizing the rubber. Raw rubber alone lacks the durability for most applications. Compounding is the process of mixing the base rubber with a carefully selected "recipe" of additives in large industrial mixers.
These additives can include fillers like carbon black to increase strength, plasticizers to improve flexibility, and protective agents to resist degradation from UV light or ozone.
Step 2: Mastication and Mixing
The raw rubber and additives are then fed into powerful machines that break down and soften the tough rubber polymers. This process, known as mastication, makes the material more pliable and ensures all the additives from the compounding stage are uniformly dispersed.
Step 3: Shaping the Rubber
Once the rubber compound is homogenous and pliable, it is shaped into its preliminary form. The two most common methods are extrusion and calendering.
- Extrusion involves forcing the rubber compound through a specially shaped die to create continuous lengths of a specific profile, such as tubing, seals, or weatherstripping.
- Calendering involves passing the rubber through a series of large rollers to press it into thin, uniform sheets or to apply it as a coating onto fabric.
Step 4: Vulcanization (Curing for Strength)
This is the final, irreversible chemical process that gives rubber its signature properties. The shaped rubber is placed in a heated press or autoclave, where agents like sulfur are used to create chemical cross-links between the long polymer chains.
This vulcanization process locks the polymers in place, transforming the soft, tacky compound into a strong, durable, and highly elastic finished product.
Understanding the Critical Trade-offs
The choice between rubber types and manufacturing processes involves balancing performance, cost, and safety.
Natural vs. Synthetic Properties
There is no single "best" rubber. Natural rubber offers excellent tensile strength and elasticity, making it ideal for things like vehicle tires. However, synthetic rubbers provide far superior resistance to oils, chemicals, and extreme temperatures.
Specialization Drives Cost
The more specialized the performance requirements, the more complex and expensive the compounding and manufacturing process becomes. A simple rubber band requires a far less rigorous process than a food-grade seal for a dairy processing plant.
Inherent Process Hazards
Rubber manufacturing involves heavy machinery, high temperatures, and flammable materials. As noted by agencies like the Health and Safety Executive, strict operational protocols are essential to mitigate risks of fire, explosions, and physical injury from equipment.
A Special Case: Food-Grade Rubber
Manufacturing rubber for food-contact applications requires an additional layer of precision and control.
The Importance of Purity
The "recipe" for food-grade rubber must only include substances that are approved as non-toxic and non-leaching by regulatory bodies. The goal is to ensure that no harmful chemicals can migrate from the rubber into the food it touches.
A Process-Driven Outcome
This strict control over ingredients and processing often results in a specific final product. For example, food-grade natural rubber is typically produced as "White Rubber," a visual confirmation that it lacks many of the common industrial fillers (like black carbon) that are unsuitable for food contact.
Making the Right Choice for Your Application
Understanding the manufacturing process empowers you to select the correct material for your specific engineering goal.
- If your primary focus is high elasticity and tear resistance: Natural rubber's molecular structure often makes it the superior choice for dynamic applications like shock absorbers or tires.
- If your primary focus is resistance to oil, fuel, or chemicals: A purpose-built synthetic rubber like Nitrile (NBR) or Viton (FKM) is the only reliable option.
- If your primary focus is a food-safe or medical application: You must specify a certified food-grade or medical-grade compound that is manufactured and documented to meet strict purity standards.
Ultimately, knowing how rubber is made is knowing what it can do.
Summary Table:
| Stage | Key Process | Purpose |
|---|---|---|
| 1. Sourcing | Harvesting latex or synthesizing polymers | Obtain base raw material (Natural or Synthetic) |
| 2. Compounding | Mixing rubber with additives (fillers, plasticizers) | Customize properties like strength and flexibility |
| 3. Shaping | Extrusion or calendering | Form into sheets, tubes, or specific profiles |
| 4. Vulcanization | Heating with sulfur to create cross-links | Permanently set shape and enhance durability |
Need a reliable partner for your rubber manufacturing lab equipment? The right tools are critical for precise compounding, mixing, and vulcanization processes. KINTEK specializes in high-quality lab equipment and consumables tailored for rubber testing and R&D. From mixers to curing presses, our solutions help you achieve consistent, high-performance results. Contact our experts today to discuss how we can support your laboratory's specific needs in material development and quality control.
Visual Guide
Related Products
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Anti-Cracking Press Mold for Lab Use
- Laboratory Test Sieves and Sieving Machines
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- What are the advantages of dual extruders? Unlock Multi-Material and Soluble Support Printing
- What is the significance of compression set? Predict Material Failure and Ensure Long-Term Reliability
- What finishes are done using calendering technique? Achieve High Gloss, Embossing, and More
- What is the process of calendering in plastic processing? A Guide to High-Volume Film & Sheet Production
- What is the importance of injection moulding machine? Unlocking High-Volume, Precision Manufacturing
- What do injection molding machines make? Mass-Producing the Plastic Parts in Your Life
- What are the disadvantages of a two roll mill? Key Safety and Consistency Risks
- What is the process of dual extrusion? Unlock the Power of Multi-Material Manufacturing



















