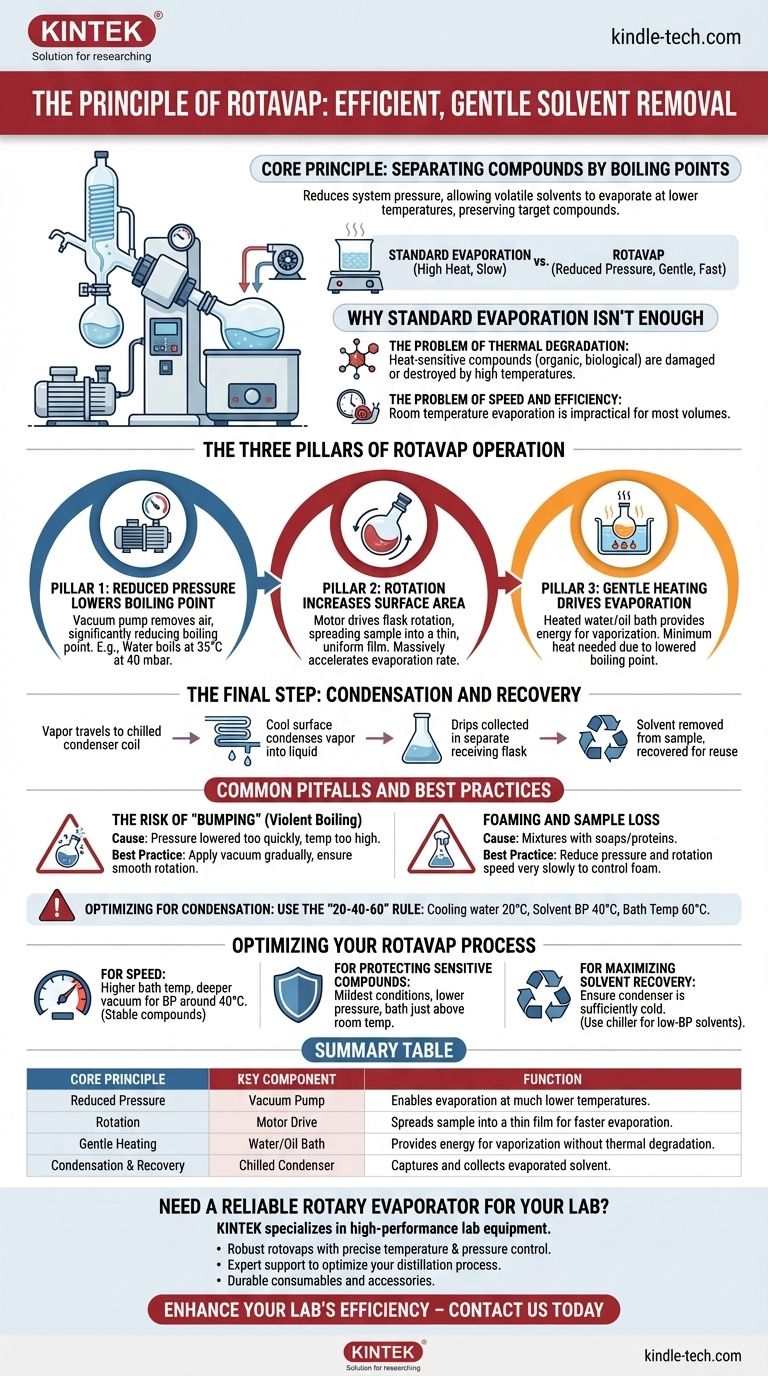
At its core, a rotary evaporator, or rotovap, separates chemical compounds based on their boiling points. It achieves this by reducing the pressure inside the system, which allows a volatile solvent to evaporate at a much lower temperature than it would at normal atmospheric pressure. This gentle evaporation process efficiently removes the solvent while preserving the integrity of the target compound left behind.
The central principle of a rotovap is that a liquid's boiling point decreases as the pressure above it decreases. The equipment leverages this physical law by combining reduced pressure, gentle heat, and flask rotation to rapidly and safely distill solvents from a sample.

Why Standard Evaporation Isn't Enough
Before understanding how a rotovap works, it's crucial to understand the problems it solves compared to simpler methods like heating a beaker on a hotplate.
The Problem of Thermal Degradation
Many organic and biological compounds are heat-sensitive. Boiling a solvent at atmospheric pressure often requires temperatures high enough to damage or completely destroy the desired, non-volatile substance you are trying to isolate.
The Problem of Speed and Efficiency
Simply leaving a solvent to evaporate at room temperature is extremely slow. While gentle, it is impractical for the volumes used in most laboratory workflows. A rotovap is designed to overcome both of these fundamental challenges.
The Three Pillars of Rotovap Operation
The efficiency of a rotary evaporator comes from the synergy of three core actions: reducing pressure, increasing surface area through rotation, and applying controlled heat.
Pillar 1: Reduced Pressure Lowers the Boiling Point
The most critical component is a vacuum pump, which removes air from the apparatus. By lowering the pressure inside the system, the solvent's boiling point is significantly reduced.
For example, water boils at 100°C (212°F) at standard atmospheric pressure but boils at just 35°C (95°F) at a pressure of 40 mbar. This allows for evaporation without aggressive heating.
Pillar 2: Rotation Increases Surface Area
The motor drive continuously rotates the evaporating flask. This action spreads the sample into a thin, uniform film on the interior wall of the flask.
This dramatically increases the surface area of the liquid exposed to the vacuum and heat, which massively accelerates the rate of evaporation. It's the same principle as a wet towel drying faster when spread out versus when it's bunched up.
Pillar 3: Gentle Heating Drives Evaporation
The evaporating flask is partially submerged in a heated water or oil bath. This bath provides a consistent and gentle source of energy (the latent heat of vaporization) needed to convert the liquid solvent into a gas.
Because the boiling point has already been lowered by the vacuum, this heat can be kept to a minimum, protecting the sample.
The Final Step: Condensation and Recovery
As the solvent evaporates, the vapor travels into a chilled condenser coil. The cool surface causes the vapor to condense back into a liquid, where it drips down and is collected in a separate receiving flask. This not only removes the solvent from the sample but also allows it to be recovered for reuse or proper disposal.
Common Pitfalls and Best Practices
While highly effective, operating a rotovap requires an understanding of its potential issues to ensure a safe and successful separation.
The Risk of "Bumping"
Bumping is the sudden, violent boiling of a liquid. This can happen if the pressure is lowered too quickly or the temperature is too high, causing you to lose a portion of your valuable sample as it splashes into the condenser.
To prevent this, always apply the vacuum gradually and ensure the flask rotation is smooth and stable before lowering it into the heat bath.
Foaming and Sample Loss
Some mixtures, especially those containing soaps or proteins, have a tendency to foam under vacuum. This foam can easily travel into the condenser, contaminating your recovered solvent and causing sample loss.
If your sample foams, you must reduce the pressure and rotation speed very slowly to keep it under control.
Maintaining the Proper Temperature Gradient
For efficient condensation, a temperature differential is key. A common rule of thumb is the "20-40-60" rule: if your cooling water is 20°C, the solvent's boiling point under vacuum should be around 40°C, and the heating bath should be set to about 60°C.
Optimizing Your Rotovap Process
How you set your parameters depends entirely on your goal. There is no single "correct" setting; there is only the best setting for your specific application.
- If your primary focus is speed: Use a higher bath temperature and a deeper vacuum that brings the solvent's boiling point down to around 40°C. This aggressive approach is suitable for stable compounds.
- If your primary focus is protecting a highly sensitive compound: Use the mildest conditions possible. Lower the pressure significantly and use a bath temperature that is only a few degrees warmer than room temperature.
- If your primary focus is maximizing solvent recovery: Ensure your condenser is sufficiently cold to capture all of the vapor. For very low-boiling-point solvents like dichloromethane, a chiller set to a low temperature is far more effective than standard tap water.
By understanding these core principles, you can confidently control the separation process, protecting your sample while achieving a fast and efficient distillation.
Summary Table:
| Core Principle | Key Component | Function |
|---|---|---|
| Reduced Pressure Lowers Boiling Point | Vacuum Pump | Enables evaporation at much lower temperatures |
| Rotation Increases Surface Area | Motor Drive | Spreads sample into a thin film for faster evaporation |
| Gentle Heating Drives Evaporation | Water/Oil Bath | Provides energy for vaporization without thermal degradation |
| Condensation and Recovery | Chilled Condenser | Captures and collects evaporated solvent for reuse or disposal |
Need a Reliable Rotary Evaporator for Your Lab?
KINTEK specializes in high-performance lab equipment, including rotary evaporators designed for efficient, gentle solvent removal. Whether you're working with heat-sensitive compounds or need to maximize solvent recovery, our solutions ensure precise control and reliable results.
We provide:
- Robust rotovaps with precise temperature and pressure control.
- Expert support to help you optimize your distillation process.
- Durable consumables and accessories for long-term performance.
Enhance your lab's efficiency and protect your valuable samples—contact us today to find the perfect rotary evaporator for your needs!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- 30T 40T Split Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What types of gases can a water circulating vacuum pump handle? Safely Manage Flammable, Condensable & Dirty Gases
- How does the impeller rotation affect the gas flow in a water circulating vacuum pump? A Guide to the Liquid Ring Principle
- Why is a water circulating vacuum pump suitable for handling flammable or explosive gases? Inherent Safety Through Isothermal Compression
- What is the importance of a vacuum pump for Schottky hybrid interfaces? Achieve Atomic-Level Purity and Bonding
- How is a circulating water vacuum pump utilized for hydrogen production residues? Optimize Your Solid-Liquid Separation



















