At its core, an autoclave test serves one critical purpose: to verify that the sterilization process is effective and reliable. It provides documented proof that an autoclave is successfully killing all microorganisms, including the most resistant bacterial spores, ensuring the safety of materials for medical, laboratory, or industrial use.
An autoclave is presumed to sterilize, but a test is required to prove it. This validation moves beyond assumption to provide certainty, confirming that the essential conditions of time, temperature, and steam saturation have been met throughout the entire load.

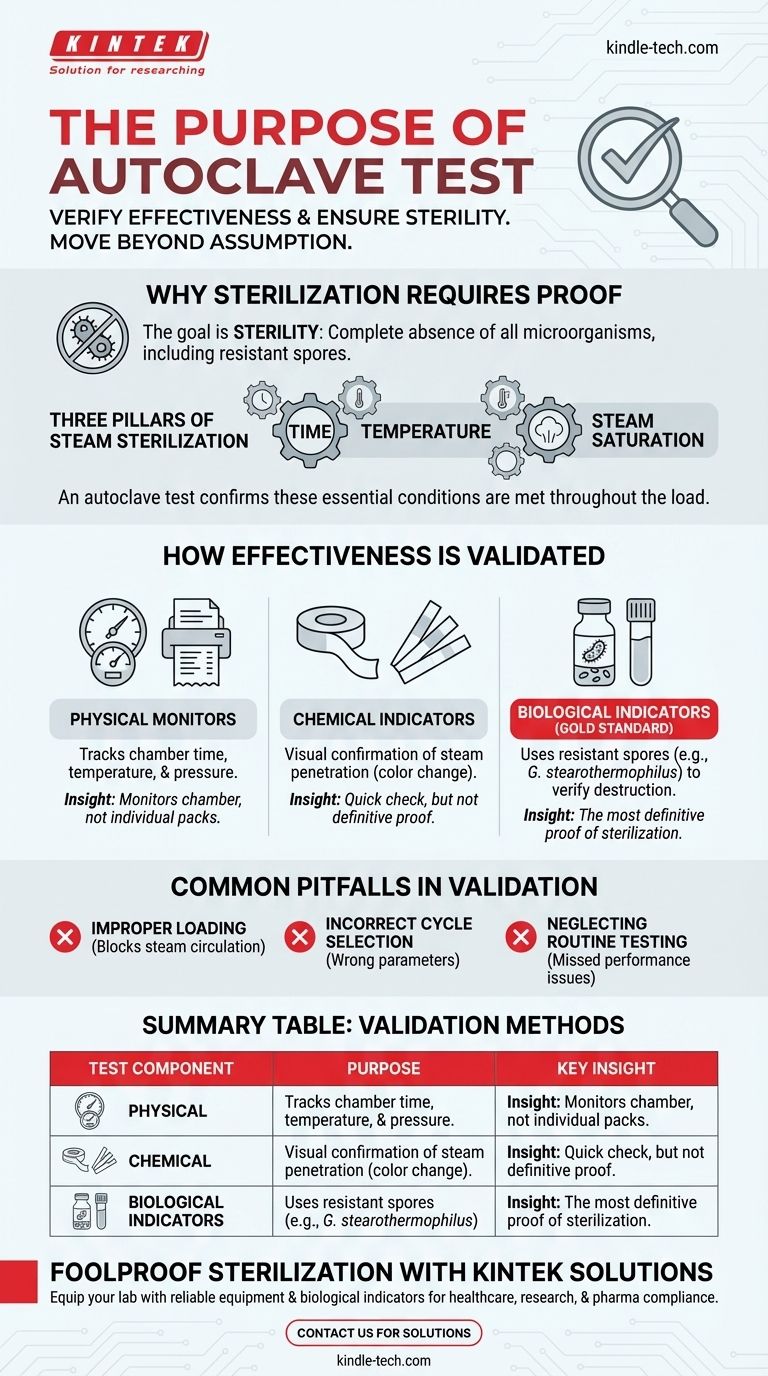
Why Sterilization Requires Absolute Proof
The primary function of an autoclave is to achieve sterility—the complete absence of viable microorganisms. This is a far higher standard than simple cleaning or disinfection.
The Standard of Sterility
Sterilization means the absolute destruction of all life, from common bacteria and viruses to highly resilient fungal and bacterial spores.
Because the consequences of a sterilization failure can be catastrophic—leading to infection, contaminated research, or unsafe products—simply running the machine is not enough. You must have objective proof that the process worked as intended.
The Three Pillars of Steam Sterilization
An autoclave achieves sterilization by applying pressurized steam at a high temperature for a specific duration. A successful cycle depends on all three factors working in concert.
An autoclave test, or validation, is designed to confirm that every item, even in the most challenging-to-reach locations within the chamber, was exposed to these lethal conditions.
How Autoclave Effectiveness Is Validated
Validation is not a single action but a multi-faceted process. It typically involves a combination of physical, chemical, and biological monitors to provide a complete picture of the autoclave's performance.
Physical Monitors
These are the readouts on the autoclave itself, such as gauges, printouts, and digital displays that track the time, temperature, and pressure for a given cycle.
While essential for monitoring, they only report conditions inside the chamber, not inside the individual packs or instruments being sterilized.
Chemical Indicators
These indicators use sensitive chemicals that change color when specific temperatures or other conditions have been met.
They are often placed on the outside (e.g., autoclave tape) and inside of each package to verify that steam has penetrated the load. They provide a quick visual confirmation but are not definitive proof of sterilization.
Biological Indicators (The Gold Standard)
The most definitive method for testing an autoclave involves using biological indicators (BIs). These are standardized vials or strips containing a high concentration of highly resistant bacterial spores, such as Geobacillus stearothermophilus.
These spores are far more difficult to kill than common microbes. If a test cycle successfully kills all the spores in the BI, it provides a high degree of confidence that all other potential contaminants in the load have also been eliminated.
Common Pitfalls in Autoclave Validation
Effective testing is not just about using the right tools; it's about avoiding common mistakes that can lead to a false sense of security.
Improper Loading
Overloading the autoclave chamber or packing items too densely is the most common cause of sterilization failure. It prevents steam from circulating freely and reaching every surface.
Incorrect Cycle Selection
Different materials require different cycle parameters. Using a "liquids" cycle for dry goods or a "gravity" cycle for complex instruments can result in incomplete sterilization.
Neglecting Routine Testing
Validation is not a one-time event. Regular testing—often weekly—with biological indicators is crucial for ensuring the machine continues to operate correctly over time and for meeting regulatory requirements.
Making the Right Choice for Your Goal
Your approach to autoclave testing should be guided by your primary objective.
- If your primary focus is patient safety in a clinical setting: You must use biological indicators for routine monitoring of every sterilizer, in addition to chemical indicators inside every critical package.
- If your primary focus is research integrity in a laboratory: Use chemical indicators in every run to prevent cross-contamination and perform regular biological indicator tests to ensure media and equipment are truly sterile.
- If your primary focus is regulatory compliance in pharmaceuticals: Your validation protocol must be rigorously documented, including initial qualification (IQ/OQ/PQ) and routine performance monitoring with BIs according to a strict schedule.
Ultimately, consistent and thorough testing is what transforms autoclaving from a routine process into a scientific guarantee of sterility.
Summary Table:
| Test Component | Purpose | Key Insight |
|---|---|---|
| Physical Monitors | Track time, temperature, and pressure inside the chamber. | Essential for cycle monitoring but doesn't confirm sterility of individual items. |
| Chemical Indicators | Provide visual confirmation (color change) that steam has penetrated the load. | Quick check for steam exposure, but not definitive proof of sterilization. |
| Biological Indicators (BIs) | Use resistant spores (e.g., Geobacillus stearothermophilus) to verify microbial destruction. | Gold standard for proving sterilization effectiveness. |
Ensure your lab's sterilization process is foolproof with KINTEK's autoclave solutions. Whether you're in healthcare, research, or pharmaceuticals, our equipment and consumables—including biological indicators and validation tools—help you meet rigorous safety and compliance standards. Contact us today to discuss your sterilization needs and protect your work with guaranteed reliability!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
People Also Ask
- What role does an autoclave play in remediation experiments? Ensure Precision by Eliminating Biological Noise
- What is the necessity of using a steam autoclave for dental alloys? Ensure Pure Bacterial Adhesion Data
- Why is a 24-hour hydrothermal treatment in an autoclave necessary for BMO nanosheets? Unlock Superior Photocatalysis
- What is the primary purpose of an autoclave in the preparation of media for the biological leaching of uranium?
- How should solid materials in bags be prepared for decontamination? Master Steam Penetration for Safe Sterilization



















