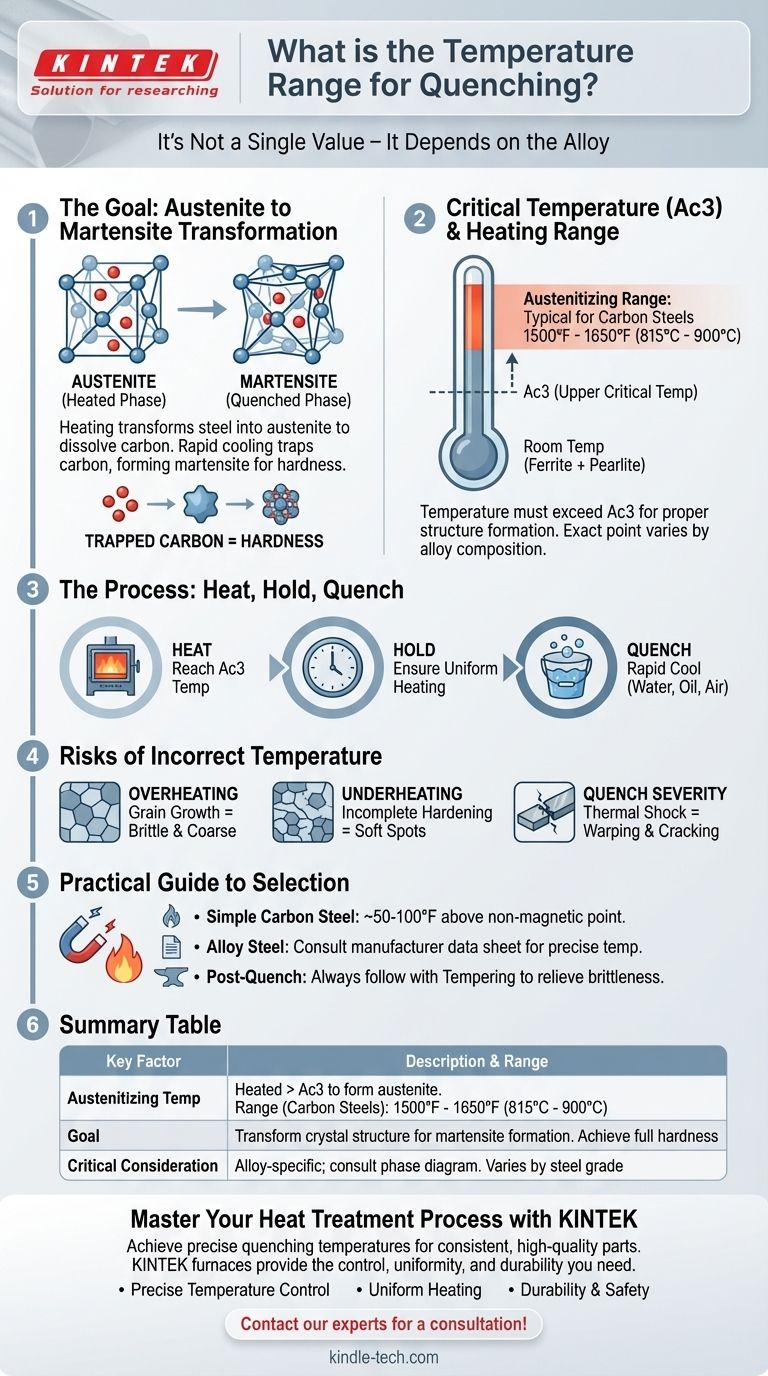
The correct temperature range for quenching is not a single value but is fundamentally dependent on the specific metal alloy being heat-treated. For common high-carbon steels, this process involves heating the metal to its austenitizing temperature, typically between 1500°F and 1650°F (815°C to 900°C), holding it there briefly, and then cooling it rapidly. The precise temperature is critical and must be above the alloy's upper critical temperature (Ac3) to ensure the proper internal structure is formed before the quench.
The goal of heating is not to reach an arbitrary temperature, but to transform the steel's internal crystal structure into a phase called austenite. Only from this state can rapid cooling, or quenching, produce the extremely hard martensite structure that is typically the goal of the process.

The "Why" Behind the Temperature: Achieving Austenite
To properly harden steel, you must first change its internal crystal lattice. This is the entire purpose of heating it before the quench.
Reaching the Critical Temperature (Ac3)
At room temperature, steel exists as a mix of ferrite and cementite (often in a structure called pearlite). This structure is relatively soft. When you heat the steel past its upper critical temperature (Ac3), this lattice transforms into a new structure called austenite.
Think of it like dissolving sugar in water. You need hot water (the austenitic phase) to dissolve a large amount of sugar (carbon). If the water isn't hot enough, the sugar won't dissolve properly.
The Austenitic Phase
Austenite is a face-centered cubic (FCC) iron structure. Its key feature is its ability to dissolve a significant amount of carbon into the iron matrix. Getting all the available carbon into this solid solution is the essential prerequisite for achieving full hardness during the quench.
Why Temperature Varies by Alloy
The exact temperature at which the austenite transformation completes (the Ac3 point) is determined by the steel's composition. Adding alloying elements like chromium, manganese, or molybdenum changes this critical temperature.
This is why a simple carbon steel like 1095 has a different austenitizing temperature than an alloy steel like 4140. The definitive source for this information is the phase diagram or technical data sheet for your specific alloy.
From Austenite to Martensite: The Quench
Once the steel is fully austenitic, the cooling process begins. The speed of this cooling is just as important as the initial temperature.
The Role of Rapid Cooling
The goal of quenching is to cool the steel so quickly that the dissolved carbon atoms do not have time to precipitate out and form softer structures like pearlite. The rapid drop in temperature effectively traps the carbon within the iron lattice.
The Martensite Transformation
As the steel cools rapidly, the iron atoms try to shift back to their room-temperature crystal structure. However, the trapped carbon atoms prevent this, forcing the lattice into a highly strained and distorted shape known as body-centered tetragonal (BCT).
This new structure, martensite, is exceptionally hard and brittle due to the immense internal stress. This hardness is the primary goal of quenching. The brittleness is a side effect that is managed later through a process called tempering.
The Quench Medium Matters
The rate of cooling is controlled by the quenching medium. Water cools fastest, followed by oil, and then air. The choice of medium depends on the steel's hardenability—its ability to form martensite. Using too aggressive a quench (like water for an oil-quenching steel) can cause warping or cracking.
Understanding the Trade-offs and Risks
Heating and cooling steel is a precise science. Getting the temperature wrong, even slightly, has significant consequences for the final product.
Risk 1: Overheating (Grain Growth)
Heating the steel too far above its Ac3 temperature, or holding it at temperature for too long, causes the individual austenite crystal grains to grow. Large grains result in a final product that is coarse and brittle, even after tempering. This damage is irreversible.
Risk 2: Underheating (Incomplete Hardening)
If you fail to heat the steel fully into the austenite phase, you will have soft spots (untransformed ferrite) remaining in the structure. When quenched, the result is a part with inconsistent hardness, as only a portion of the steel transformed into martensite.
Risk 3: Cracking and Warping
The transformation to martensite involves a slight expansion of the steel's volume. This, combined with the thermal shock of the quench, creates massive internal stress. If the quench is too severe for the alloy or the part has sharp internal corners, these stresses can cause the part to warp significantly or crack.
A Practical Guide to Selecting Your Quenching Temperature
Your approach should be dictated by the specific material you are working with and your desired outcome.
- If your primary focus is hardening a simple carbon steel (e.g., 1084, 1095): Heat the steel to about 50-100°F (30-55°C) above the point where it becomes non-magnetic, typically targeting a range of 1500-1550°F (815-845°C).
- If your primary focus is hardening an alloy steel (e.g., 4140, 5160, O1): You must consult the manufacturer's technical data sheet for the precise austenitizing temperature, as it can be significantly different from carbon steels.
- If your primary focus is achieving a tough, durable part: Remember that quenching is only the first step; it must be followed by tempering to relieve brittleness and achieve the final desired balance of hardness and toughness.
Ultimately, mastering quenching is about precisely controlling the steel's internal crystal structure, not just its temperature.
Summary Table:
| Key Factor | Description | Typical Range for Carbon Steels |
|---|---|---|
| Austenitizing Temperature | Heated above the upper critical temperature (Ac3) to form austenite | 1500°F - 1650°F (815°C - 900°C) |
| Goal | Transform crystal structure to enable martensite formation upon rapid cooling | Achieve full hardness |
| Critical Consideration | Temperature is alloy-specific; consult the material's phase diagram | Varies by steel grade |
Master Your Heat Treatment Process with KINTEK
Achieving the precise quenching temperature is critical for the performance and durability of your metal parts. Inconsistent heating can lead to soft spots, warping, or cracking, compromising your entire production run.
KINTEK specializes in high-performance lab furnaces and ovens that deliver the exact, uniform temperatures required for perfect quenching results. Our equipment is trusted by metallurgists and manufacturers for its reliability and precision.
We provide solutions for:
- Precise Temperature Control: Ensure your steel reaches and holds the correct austenitizing temperature.
- Uniform Heating: Eliminate cold spots for consistent material properties throughout your part.
- Durability and Safety: Built to withstand rigorous industrial use.
Don't leave your heat treatment results to chance. Let KINTEK's expertise in laboratory heating equipment help you achieve perfect hardness and superior part quality every time.
Contact our experts today for a personalized consultation on the ideal furnace for your quenching application!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the difference between liquid and gas carburizing? Precision, Safety & Environmental Impact
- Which furnace is used for heat treatment? A Guide to Choosing the Right Furnace for Your Materials
- Why is temperature important in casting? Master the Thermal Balance for Defect-Free Parts
- Why is a vacuum environment system necessary for SEP of CuAlMn alloys? Achieve High-Purity Porous Structures
- What temperature is a hardening furnace? Achieve Precise Heat Treatment for Superior Metal Hardness
- Can I melt aluminum on the stove? Why It's a Dangerous and Ineffective Method
- What is the primary purpose of using an electric drying oven for dense refractory bricks? Optimize Raw Material Prep
- What materials can be tempered? Unlock the Key to Hardness and Toughness in Steel



















