At its core, the theory of thin film evaporation is about dramatically increasing the rate of heat transfer and reducing the thermal stress on a substance. This is achieved by mechanically spreading the liquid into a very thin, turbulent film across a heated surface, often under a vacuum, allowing for nearly instantaneous evaporation of volatile components.
The fundamental goal is not simply to boil a liquid, but to separate its components with maximum speed and minimum heat damage. Thin film evaporation masters this by manipulating surface area and pressure to create a process that is both highly efficient and exceptionally gentle.

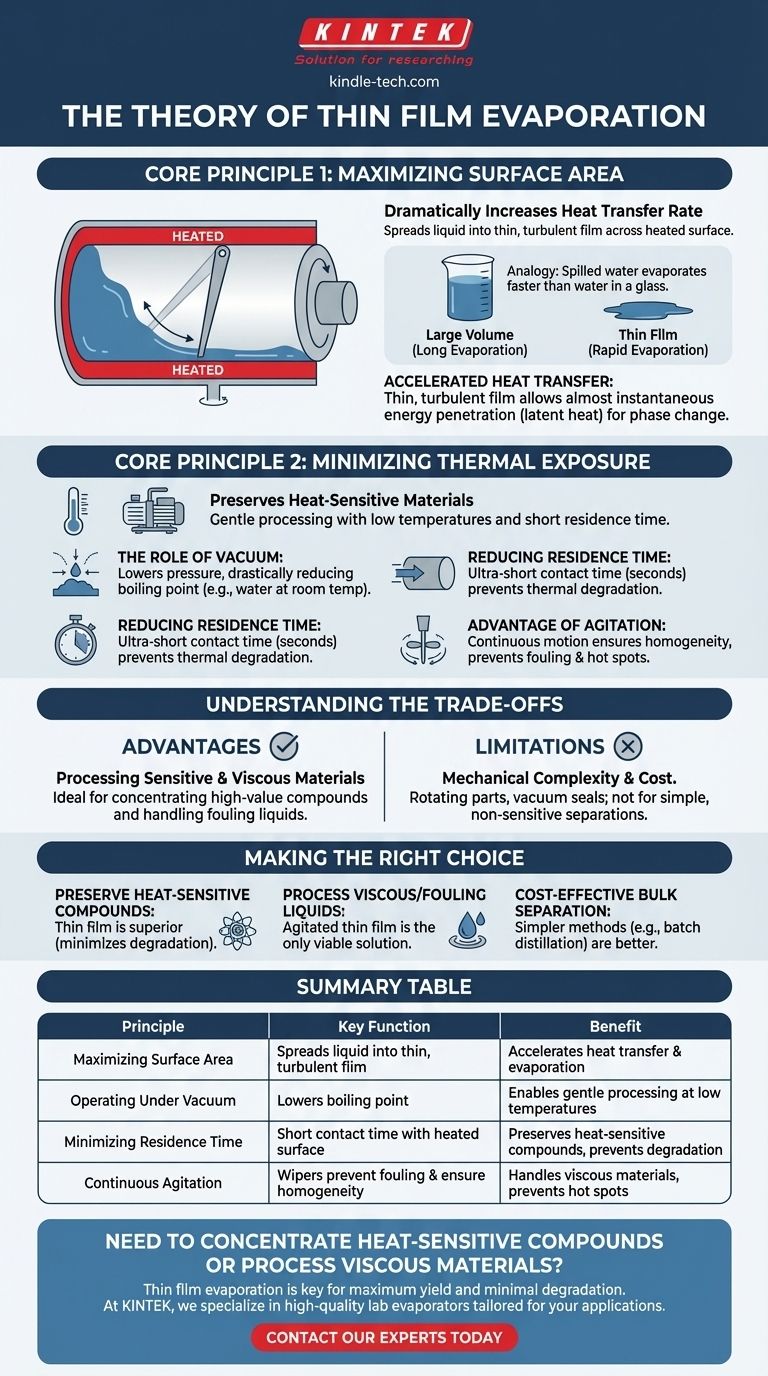
The Core Principle: Maximizing Surface Area
The efficiency of evaporation is directly tied to how quickly you can transfer heat into a liquid and how easily the resulting vapor can escape.
Creating the Thin Film
In a thin film evaporator, the feed liquid is not boiled in a large pot. Instead, it is distributed as a very thin layer across the inner wall of a heated cylinder.
This is typically accomplished using a system of rotating blades, wipers, or rollers that continuously spread the liquid. In a lab-scale rotary evaporator, the rotation of the flask itself achieves this effect.
The Power of a Large Surface-to-Volume Ratio
By spreading the liquid out, you radically increase the surface area exposed to heat relative to the total volume.
Think of spilling a glass of water on a hot sidewalk. The thin puddle evaporates in seconds, while the same amount of water in the glass would take hours to evaporate. Thin film technology applies this exact principle in a controlled environment.
Accelerating Heat Transfer
A thin, turbulent film has an extremely low resistance to heat transfer. The energy from the heated wall can penetrate the entire liquid layer almost instantly, providing the necessary energy (latent heat of vaporization) for the phase change to occur rapidly.
The Second Principle: Minimizing Thermal Exposure
For many materials in the pharmaceutical, food, and specialty chemical industries, heat is the enemy. Prolonged exposure, even to moderate temperatures, can cause degradation, loss of potency, or undesirable side effects.
The Role of Vacuum
Thin film evaporators almost always operate under a deep vacuum. Lowering the pressure inside the system drastically reduces the boiling point of the liquid.
For example, water boils at 100°C (212°F) at sea level, but under a strong vacuum, it can boil at room temperature. This allows for evaporation to occur at much lower, safer temperatures.
Reducing Residence Time
Because evaporation is so rapid, the amount of time the material spends inside the heated evaporator (its residence time) is incredibly short—often just a matter of seconds.
This combination of low temperature (due to vacuum) and short residence time is the key to gently processing thermally sensitive materials without damaging them.
The Advantage of Agitation
The constant motion from the rotating wipers does more than just create the film. It provides constant agitation, ensuring the film is homogenous and preventing any single portion of the liquid from getting stuck to the wall and overheating (known as "fouling" or "hot spots").
This is especially critical for viscous or heat-sensitive products that would quickly burn or degrade in a standard evaporator.
Understanding the Trade-offs
No technology is perfect for every application. Understanding the limitations is as important as knowing the benefits.
Key Advantage: Processing Sensitive & Viscous Materials
The ability to operate at low temperatures with short residence times makes this technology ideal for concentrating or separating high-value, heat-sensitive compounds. The mechanical wiping action also allows it to process viscous or fouling liquids that are impossible to handle in static systems.
Limitation: Mechanical Complexity and Cost
These systems involve rotating internal parts, complex vacuum seals, and precise engineering. This makes them significantly more expensive and mechanically complex to maintain than a simple batch distillation column or pot evaporator.
Limitation: Not Ideal for Simple Separations
If you are separating non-sensitive, low-viscosity materials (like separating salt from water), the complexity and cost of a thin film evaporator are unnecessary. A simpler, more cost-effective technology would be sufficient.
Making the Right Choice for Your Goal
Your decision to use thin film evaporation must be based on the properties of your material and the desired outcome.
- If your primary focus is preserving heat-sensitive compounds: Thin film evaporation is the superior method because it minimizes thermal degradation by lowering the boiling point and reducing residence time.
- If your primary focus is processing viscous or fouling liquids: The mechanical action of an agitated thin film evaporator is often the only viable solution to ensure efficient heat transfer and prevent product buildup.
- If your primary focus is cost-effective bulk separation of non-sensitive materials: A simpler method like batch distillation is likely a more practical and economical choice.
By understanding the interplay of surface area, pressure, and time, you can effectively leverage evaporation technology to meet your specific processing needs.
Summary Table:
| Principle | Key Function | Benefit |
|---|---|---|
| Maximizing Surface Area | Spreads liquid into a thin, turbulent film | Drastically accelerates heat transfer and evaporation rate |
| Operating Under Vacuum | Lowers the boiling point of the liquid | Enables gentle processing at low temperatures |
| Minimizing Residence Time | Short contact time with heated surface | Preserves heat-sensitive compounds and prevents degradation |
| Continuous Agitation | Wipers prevent fouling and ensure homogeneity | Handles viscous materials and prevents hot spots |
Need to concentrate heat-sensitive compounds or process viscous materials efficiently? The theory of thin film evaporation is key to achieving your goals with maximum yield and minimal degradation. At KINTEK, we specialize in providing high-quality lab equipment, including evaporators tailored for demanding laboratory applications. Contact our experts today to find the perfect solution for your separation and concentration challenges!
Visual Guide
Related Products
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Electron Beam Evaporation Coating Conductive Boron Nitride Crucible BN Crucible
People Also Ask
- How is deposition time calculated? Mastering the Clock for Strategic Legal Advantage
- What are the uses of evaporation in industry? From Food Concentration to High-Tech Thin Films
- What is the purpose of vacuum evaporation? Purify Water or Create High-Purity Coatings
- What is the thermal evaporation technique? A Guide to Thin-Film Deposition for Your Lab
- What is the vacuum level of a thermal evaporator? Achieve Purity with High Vacuum (10⁻⁵ to 10⁻⁷ Torr)



















