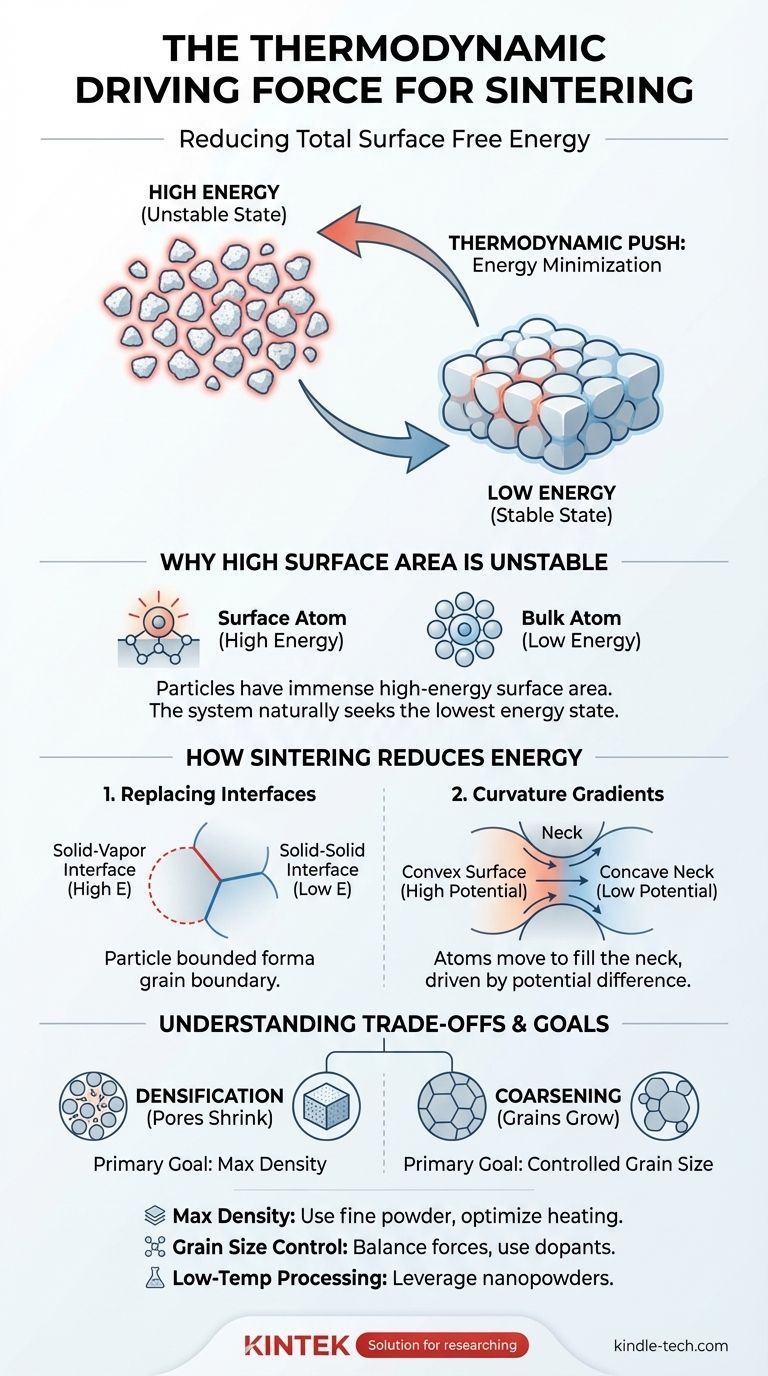
The fundamental thermodynamic driving force for sintering is the reduction of the total surface free energy of a system. A collection of individual particles possesses an enormous amount of high-energy surface area compared to a single, dense solid of the same mass. Sintering is the thermally activated process by which the system lowers its overall energy by replacing these high-energy solid-vapor interfaces with lower-energy solid-solid interfaces, causing the particles to bond together.
Sintering is fundamentally a process of energy minimization. A collection of fine particles exists in a high-energy, unstable state. The system naturally seeks to reduce this excess surface energy by bonding particles together, forming a denser, more stable structure.

Why High Surface Area is Unstable
To grasp the driving force, we must first understand why a powder is inherently less stable than a solid block.
The Concept of Surface Energy
Atoms within the bulk of a material are bonded to their neighbors on all sides, creating a stable, low-energy state.
Atoms on a surface, however, have fewer neighboring atoms to bond with. This lack of complete bonding leaves them in a higher, more unfavorable energy state. This excess energy at the surface is called surface energy or surface tension.
A System's Goal: Energy Minimization
Like a ball rolling downhill, all physical systems naturally tend toward their lowest possible energy state.
For a collection of powder particles, the state of lowest energy is a single, dense solid with minimal surface area. The vast surface area of the powder represents a significant amount of stored potential energy, which provides the thermodynamic "push" for sintering to occur.
How Sintering Reduces System Energy
Sintering is the pathway the material takes to release this stored surface energy. It does so by changing the system's geometry at the atomic level.
Replacing High-Energy Surfaces
The core of the process involves eliminating the high-energy solid-vapor interfaces (the particle surfaces) and replacing them with lower-energy solid-solid interfaces, which we know as grain boundaries.
While grain boundaries also represent an increase in energy over a perfect single crystal, the energy of this new interface is significantly lower than the energy of the two free surfaces it replaced.
The Role of Curvature Gradients
The driving force is most intense at the points of contact between particles. These contact points form small, concave "necks."
Atoms on the convex surfaces of the particles have a higher chemical potential (are in a higher energy state) than atoms in the concave neck region. This difference in potential, or curvature gradient, drives the net diffusion of atoms from the particle surfaces into the growing neck.
The Result: Densification
As atoms move to form and grow these necks, the centers of the particles draw closer together.
This microscopic action results in the macroscopic shrinkage of the powder compact and the elimination of the pores between particles. This process is known as densification.
Understanding the Trade-offs
The driving force explains why sintering happens, but it doesn't happen in isolation. It's crucial to distinguish the driving force from the mechanisms that enable it.
Driving Force vs. Atomic Transport
The reduction in surface energy is the thermodynamic reason for sintering. However, for it to actually occur, atoms must physically move.
This movement happens through various atomic transport mechanisms (e.g., surface diffusion, grain boundary diffusion), which are only activated with sufficient thermal energy (heat). Without heat, the driving force exists, but the atoms lack the mobility to act on it.
The Competing Process: Coarsening
Densification is not the only process that reduces system energy. Coarsening, or grain growth, also occurs.
During coarsening, larger grains grow at the expense of smaller ones, which reduces the total area of grain boundaries in the system. This also lowers the system's energy but does not necessarily increase its density. Managing the balance between densification and coarsening is a primary challenge in materials processing.
Making the Right Choice for Your Goal
Understanding this fundamental driving force allows you to manipulate the sintering process to achieve specific material outcomes.
- If your primary focus is achieving maximum density: Your goal is to use processing conditions (like smaller initial particle size and specific heating profiles) that maximize the driving force for densification while minimizing the transport mechanisms that lead to coarsening.
- If your primary focus is controlling final grain size: You must carefully balance the driving force for densification against the driving force for grain growth, often by using dopants to pin grain boundaries or by employing advanced techniques like field-assisted sintering.
- If your primary focus is low-temperature processing: You must maximize the initial driving force by using nanopowders, which have exceptionally high surface area and are therefore much more thermodynamically driven to sinter at lower temperatures.
Ultimately, viewing sintering as a system's relentless effort to shed its excess surface energy is the key to intelligently controlling the final properties of your material.
Summary Table:
| Key Concept | Role in Sintering Driving Force |
|---|---|
| Surface Energy | High-energy state of surface atoms creates instability in powders. |
| Energy Minimization | The system's natural tendency to move to a lower energy state. |
| Solid-Vapor to Solid-Solid | Replacing high-energy particle surfaces with lower-energy grain boundaries. |
| Curvature Gradients | Creates a chemical potential difference that drives atomic diffusion into necks between particles. |
Ready to harness the principles of sintering to optimize your materials processing?
At KINTEK, we specialize in providing the advanced lab equipment and expert support you need to master sintering for your specific application—whether your goal is maximum density, controlled grain size, or low-temperature processing. Our range of sintering furnaces and consumables is designed to help you achieve precise, repeatable results.
Contact our experts today to discuss how we can help you unlock the full potential of your materials.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory Quartz Tube Furnace with Alumina Tube Tubular Furnace
People Also Ask
- What are the disadvantages of a muffle furnace? Understanding the Trade-offs for Your Lab
- How accurate is the muffle furnace? Achieve ±1°C Control and ±2°C Uniformity
- What are the conditions for a muffle furnace? Ensure Safety, Performance, and Longevity
- What is the difference between muffle furnace and air oven? Choose the Right Tool for Your Thermal Process
- What is the difference between a box furnace and a muffle furnace? Choose the Right Lab Furnace for Your Application



















