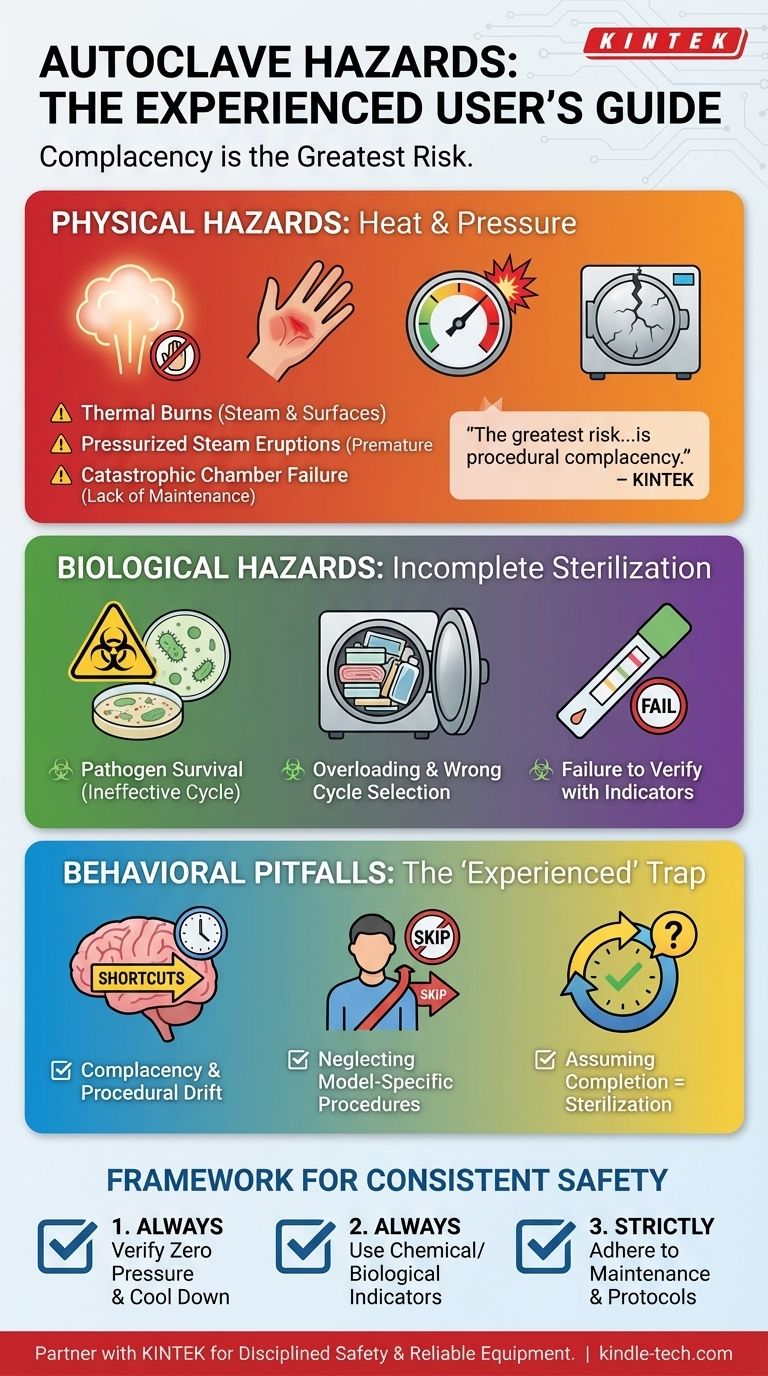
Even for an experienced operator, the primary hazards associated with an autoclave fall into three categories: severe physical injury from heat and pressure, biological exposure from incomplete sterilization, and potential chemical reactions. The most common and immediate dangers are thermal burns from superheated steam and surfaces, and violent eruptions of steam and hot water from opening the door prematurely.
The greatest risk for an experienced lab professional is not equipment failure, but procedural complacency. Hazards arise when familiarity leads to shortcuts in loading, unloading, and respecting the essential cooling-down period.

The Primary Physical Hazards: Heat and Pressure
Even a routine autoclave cycle involves extreme temperatures and high pressure, creating a constant potential for severe physical injury if protocols are not strictly followed.
Thermal Burns
The most frequent autoclave-related injury is a thermal burn. These can occur from touching the chamber door or walls, handling items before they have adequately cooled, or exposure to superheated steam.
Steam that is released from the chamber, even if invisible, is still at or above 100°C (212°F) and can cause immediate, severe burns upon contact with skin. This is why heat-resistant gloves and other appropriate PPE are non-negotiable.
Pressurized Steam Eruptions
A far more dangerous hazard is the explosive release of steam and boiling water from opening the chamber door too soon. If the chamber is still pressurized, even slightly, the door can be thrown open violently.
This event can cause a sudden and large-scale eruption of boiling water and steam, leading to severe burns over a large area of the body. Always wait for the pressure gauge to read zero and allow the prescribed cooling time before attempting to open the door.
Catastrophic Chamber Failure
While rare, it is possible for the autoclave chamber itself to fail or explode. This is typically caused by a malfunctioning safety valve, a blocked vent, or improper loading that prevents steam from circulating correctly, leading to a dangerous pressure buildup.
Regular maintenance, inspection, and proper record-keeping are critical to ensuring all safety mechanisms are functioning as designed.
The Unseen Biological Hazard: Incomplete Sterilization
The entire purpose of an autoclave is to sterilize materials. A failure in this process can expose personnel to viable and dangerous pathogens.
The Risk of Pathogen Survival
If a sterilization cycle is ineffective, items removed from the autoclave are not sterile. Handling these items under the assumption they are safe creates a significant risk of infection or contamination.
This is a subtle but critical hazard, as the items may appear "processed" but still harbor dangerous microorganisms.
Common Causes of Failure
Sterilization failure often results from operator error. Common causes include overloading the chamber, packing items too densely, using the wrong cycle for the load type, or insufficient water levels.
A robust monitoring program, using biological or chemical indicators, is the only way to verify that each cycle achieves effective sterilization.
Common Pitfalls for the Experienced User
Experience can sometimes foster a false sense of security. The most insidious risks for a seasoned operator are not from a lack of knowledge, but from a gradual deviation from established safety protocols.
Complacency and Procedural Drift
After hundreds of successful cycles, it becomes easy to take small shortcuts—cracking the door a minute early, forgoing gloves for a "quick" unload, or skipping a log entry.
This procedural drift is a primary cause of accidents. Each step in the operating procedure exists to mitigate a specific, identified hazard.
Neglecting Model-Specific Procedures
Not all autoclaves are identical. An operator experienced with one model may make dangerous assumptions when using another.
Always reviewing the owner's manual for a specific machine is critical, as controls, safety features, and cycle parameters can vary significantly.
Assuming a Completed Cycle is a Successful Cycle
Seeing the cycle finish does not guarantee sterilization was achieved. A clogged drain or malfunctioning sensor can allow a cycle to complete without ever reaching the required temperature or pressure.
This is why physical monitoring (checking gauges and printouts) and chemical/biological indicators are essential checks against equipment malfunction.
A Framework for Consistent Safety
Your approach to autoclave operation must be built on a foundation of disciplined, consistent adherence to established protocols, regardless of your experience level.
- If your primary focus is personal physical safety: Always verify the chamber pressure is at zero and respect the full cooling period before opening the door—no exceptions.
- If your primary focus is guaranteeing effective sterilization: Focus on proper loading techniques to allow for steam penetration and use chemical or biological indicators in every load to confirm success.
- If your primary focus is long-term operational integrity: Adhere strictly to the schedule for maintenance, testing, and record-keeping to prevent catastrophic equipment failure.
Ultimately, consistent safety relies on disciplined adherence to procedure, not just on past experience.
Summary Table:
| Hazard Category | Key Risks | Primary Cause |
|---|---|---|
| Physical | Thermal burns, steam eruptions, chamber failure | Procedural shortcuts, opening door prematurely |
| Biological | Exposure to pathogens from incomplete sterilization | Overloading, wrong cycle, equipment malfunction |
| Behavioral | Complacency, procedural drift, model-specific errors | False sense of security from experience |
Ensure your lab's autoclave safety and performance with KINTEK.
As specialists in laboratory equipment and consumables, we understand that consistent safety requires reliable equipment and proper protocols. KINTEK provides high-performance autoclaves, essential safety accessories, and expert support to help your team:
- Prevent accidents with autoclaves featuring robust safety mechanisms and clear operational guidance.
- Achieve guaranteed sterilization every time with proper loading techniques and validation tools.
- Maintain long-term operational integrity through scheduled maintenance and dependable service.
Don't let experience lead to complacency—partner with KINTEK to build a foundation of disciplined safety. Contact our experts today for a consultation on the right autoclave solution for your laboratory's specific needs.
Visual Guide
Related Products
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- What are the chambers of the autoclave? Understanding Single-Wall vs. Jacketed Designs
- What are the specifications of a laboratory autoclave? A Guide to Key Features for Safe Sterilization
- What is the temperature of autoclave 132? A Guide to High-Speed Sterilization Cycles
- What is the temperature of autoclave in microbiology lab? Achieve Sterile Conditions with 121°C
- What is the risk assessment for use of autoclave? Avoid Material Damage and Sterilization Failure



















