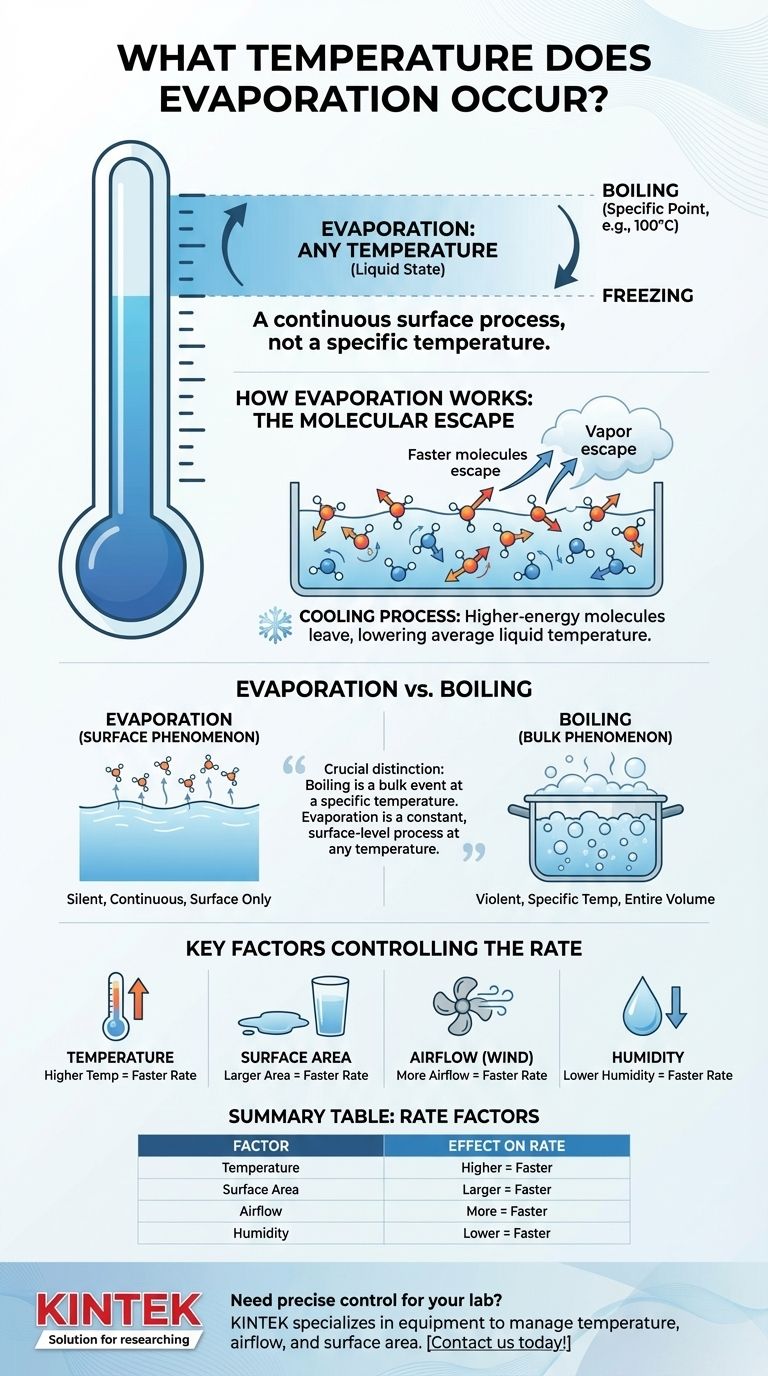
To be precise, evaporation occurs at any temperature where a substance is in its liquid state. Unlike boiling, which happens at a specific temperature (like 100°C or 212°F for water at sea level), evaporation is a continuous surface process that happens at all temperatures, from just above freezing to just below boiling.
The critical distinction is that boiling is a bulk event that happens at a specific temperature throughout the entire liquid, while evaporation is a constant, surface-level process that can occur at any temperature. The question is not at what temperature it happens, but how fast it happens under different conditions.

How Evaporation Actually Works
To understand why there isn't a single "evaporation temperature," we need to look at the behavior of molecules within a liquid.
The Constant Motion of Molecules
In any liquid, molecules are in constant, chaotic motion. They are not all moving at the same speed; there is a wide distribution of kinetic energies. Temperature is simply a measure of the average kinetic energy of these molecules.
The Escape from the Surface
At the surface of the liquid, some of the faster-moving molecules may have enough energy to overcome the attractive forces holding them to their neighbors. When they do, they escape the liquid and become a gas (vapor). This is evaporation.
Why It's a "Cooling" Process
Because only the highest-energy ("hottest") molecules are able to escape, the average energy of the remaining molecules drops. This is why evaporation is a cooling process—it’s how sweat cools your body.
The Critical Difference: Evaporation vs. Boiling
The core of this topic lies in understanding that these are two distinct ways for a liquid to become a gas.
Evaporation: The Silent Escape
Evaporation is a surface phenomenon. It happens only at the boundary between the liquid and the air above it. It occurs silently and continuously at any temperature as long as the liquid is exposed.
Boiling: The Violent Transformation
Boiling is a bulk phenomenon. It occurs throughout the entire volume of the liquid when the liquid's vapor pressure equals the surrounding atmospheric pressure. This allows bubbles of vapor to form inside the liquid and rise to the surface. This process only happens at a specific temperature known as the boiling point.
Key Factors That Control the Rate of Evaporation
While evaporation always happens, its speed can change dramatically. Your original question about temperature is important because temperature is the primary factor controlling the rate of evaporation.
Temperature
Higher temperature means faster evaporation. As you increase the temperature of a liquid, the average kinetic energy of its molecules increases, meaning more molecules have sufficient energy to escape the surface.
Surface Area
Greater surface area means faster evaporation. A puddle of water on the floor will evaporate much faster than the same amount of water in a tall glass because more molecules are exposed to the air and have a chance to escape.
Airflow (Wind)
More airflow means faster evaporation. Wind blows away the vapor that has just escaped the liquid's surface. This reduces the concentration of vapor in the air directly above the liquid, making it easier for more molecules to escape.
Humidity
Lower humidity means faster evaporation. Humidity is the amount of water vapor already in the air. If the air is already saturated (100% humidity), it can't hold any more vapor, and net evaporation stops. Dry air, by contrast, readily accepts new vapor molecules.
Common Pitfalls to Avoid
Understanding the process correctly requires sidestepping a very common mental trap.
The Myth of an "Evaporation Point"
The most frequent mistake is thinking of evaporation as an event with a trigger point, like boiling. It is not an on/off switch.
Evaporation should be understood as a rate, not a state. It is a continuous process that can be fast or slow depending on the conditions, but it is always occurring as long as a liquid surface is exposed to an environment that is not fully saturated.
Making the Right Choice for Your Goal
By understanding these principles, you can manipulate the process to achieve a specific outcome.
- If your primary focus is to dry something quickly: You must maximize the rate of evaporation by increasing temperature (using a hairdryer), increasing airflow (using a fan), and increasing surface area (spreading a towel out flat).
- If your primary focus is to preserve a liquid: You must minimize the rate of evaporation by keeping it cool, in a container with a small opening (low surface area), and covering it to stop airflow and create a pocket of high humidity.
- If your primary focus is to create a cooling effect: You need to encourage rapid evaporation by applying a thin layer of liquid (like water or alcohol) over a large surface area and exposing it to airflow.
By shifting your focus from a specific temperature to the factors controlling the rate of evaporation, you gain true control over the process.
Summary Table:
| Factor | Effect on Evaporation Rate |
|---|---|
| Temperature | Higher temperature = Faster rate |
| Surface Area | Larger area = Faster rate |
| Airflow | More airflow = Faster rate |
| Humidity | Lower humidity = Faster rate |
Need precise control over evaporation for your lab processes? KINTEK specializes in lab equipment and consumables that help you manage temperature, airflow, and surface area with precision. From heating mantles to controlled environment chambers, our solutions ensure reliable and repeatable results for your laboratory needs. Contact us today to optimize your evaporation and drying applications!
Visual Guide
Related Products
- Wall Mounted Water Distillation Unit
- Evaporation Crucible for Organic Matter
- Evaporation Boat for Organic Matter
- Tungsten Evaporation Boat for Thin Film Deposition
- High Purity Pure Graphite Crucible for Evaporation
People Also Ask
- Is THC distillate better? Discover the Pros and Cons of Pure Potency vs. Full-Spectrum Effects
- What is the delta 20 rule of evaporation? Master Safe and Effective Spraying
- Does THC distillate lose potency? A guide to preserving your product's power.
- What is the difference between cannabis extract and distillate? A Guide to Potency vs. Full-Spectrum Effects
- What are the disadvantages of distilling? The Hidden Costs of Model Compression




