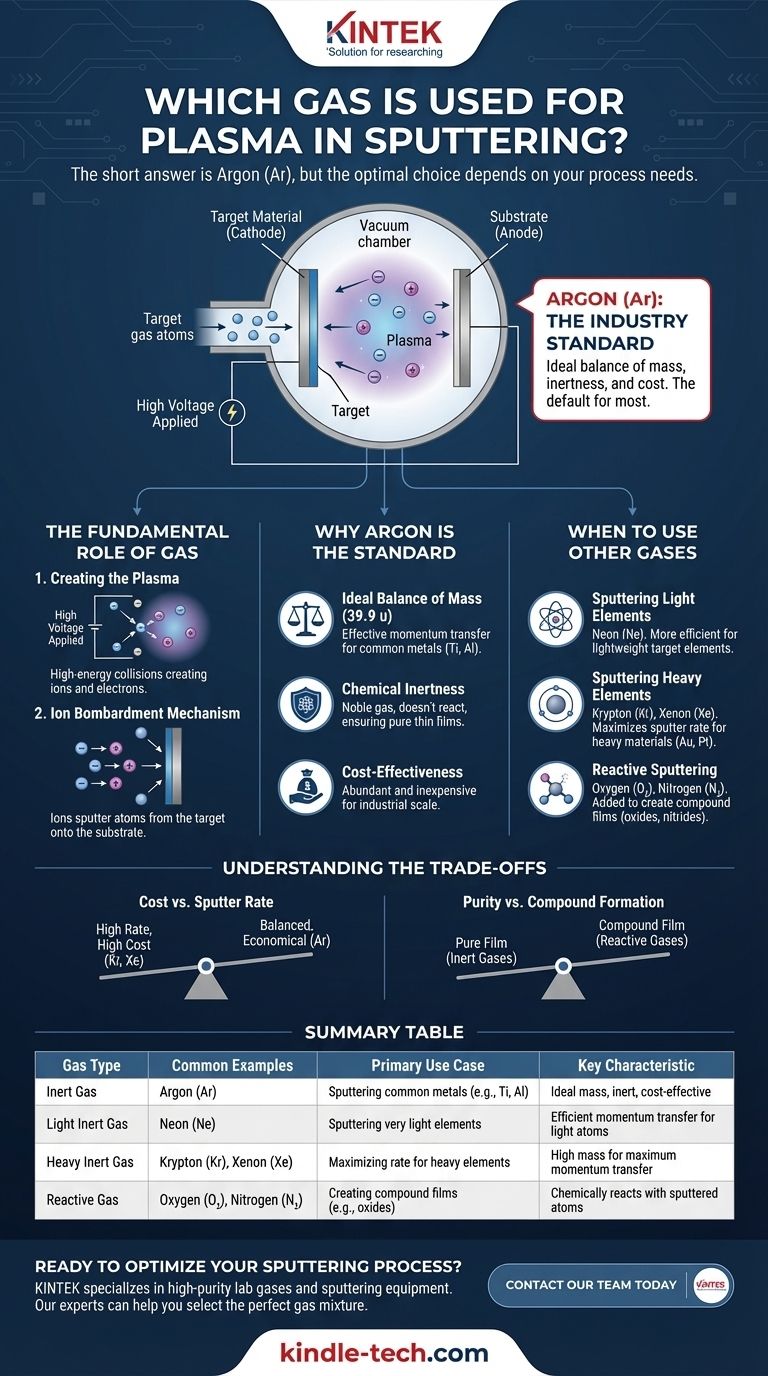
The short answer is Argon (Ar). This inert noble gas is the most common and versatile choice for generating the plasma required for sputtering. However, the selection of the right gas is a critical decision that directly influences the efficiency of the process and the chemical properties of the final thin film.
The gas used in sputtering is not merely a catalyst; it is the medium for energy transfer. While Argon is the industry standard due to its ideal balance of mass, inertness, and cost, the optimal choice depends entirely on matching the gas's atomic weight to the target material and determining if a chemical reaction is desired.

The Fundamental Role of Gas in Sputtering
To understand why a specific gas is chosen, we must first understand its function. The gas doesn't just fill the chamber; it becomes the active tool for the deposition process.
Creating the Plasma
Sputtering begins by introducing a low-pressure gas into a vacuum chamber. A high voltage is then applied between the target material (cathode) and the chamber/substrate holder (anode).
This strong electric field accelerates free electrons, causing them to collide with the neutral gas atoms. These high-energy collisions knock electrons off the gas atoms, creating a cascade of positively charged ions and free electrons—a glowing, ionized state of matter known as plasma.
The Ion Bombardment Mechanism
The newly created positive gas ions are accelerated with great force toward the negatively charged target.
Upon impact, these ions physically knock out, or "sputter," atoms from the target material. These ejected target atoms then travel through the chamber and deposit onto a substrate, forming a uniform thin film.
Why Argon is the Standard Choice
Argon is the default gas for most sputtering applications for several well-established reasons.
Ideal Balance of Mass
For sputtering to be efficient, there needs to be effective momentum transfer between the gas ion and the target atom, much like a good break in a game of pool. Argon's atomic mass (39.9 u) is a suitable match for many commonly sputtered metals, like titanium and aluminum, allowing for effective energy transfer without excessive cost.
Chemical Inertness
As a noble gas, Argon is chemically inert. It will not react with the target material during bombardment or with the deposited atoms on the substrate. This ensures the resulting thin film is a pure representation of the target material.
Cost-Effectiveness
Compared to other noble gases, Argon is abundant and relatively inexpensive, making it the most economical choice for industrial-scale production.
When to Use Other Gases: A Strategic Decision
Choosing a gas other than Argon is a deliberate decision made to optimize the process for specific materials or outcomes.
Sputtering Light Elements
When sputtering very light target elements, a lighter inert gas like Neon (Ne) may be used. Its lower atomic mass provides a more efficient "billiard ball" collision for dislodging lightweight atoms.
Sputtering Heavy Elements
Conversely, to maximize the sputtering rate of heavy elements like gold or platinum, a heavier inert gas like Krypton (Kr) or Xenon (Xe) is superior. Their greater mass transfers significantly more momentum upon impact, increasing the sputter yield.
Reactive Sputtering
Sometimes the goal is not to deposit a pure material but a compound. In reactive sputtering, gases like Oxygen (O2) or Nitrogen (N2) are intentionally added to the chamber along with Argon.
The reactive gas combines with the sputtered target atoms either in transit or on the substrate surface. This technique is essential for creating durable compound films like titanium nitride (TiN) or transparent conductive oxides.
Understanding the Trade-offs
Every gas choice involves a balance between performance and practicality.
Cost vs. Sputter Rate
The primary trade-off is cost versus efficiency. Krypton and Xenon can dramatically increase deposition rates, but their high cost can be prohibitive for many applications. The process must justify the expense through higher throughput or specific film requirements.
Purity vs. Compound Formation
The choice between an inert or reactive gas is fundamental. Using an inert gas guarantees the purity of the deposited film. Intentionally introducing a reactive gas is a calculated move to create a new material with entirely different properties from the original target.
Selecting the Right Gas for Your Application
- If your primary focus is general-purpose sputtering of common metals: Argon is the reliable, cost-effective, and technically sound default choice.
- If your primary focus is to maximize the deposition rate of a heavy element: Evaluate Krypton or Xenon, understanding that it comes with a significant increase in operational cost.
- If your primary focus is to create a specific compound film (e.g., an oxide or nitride): You must use a reactive sputtering process with a controlled mixture of Argon and a reactive gas like Oxygen or Nitrogen.
- If your primary focus is sputtering a very light element with maximum efficiency: Neon may provide a better mass match and a more efficient momentum transfer than Argon.
Ultimately, the choice of sputtering gas is a strategic decision that directly controls the efficiency, chemistry, and cost of your thin-film deposition process.
Summary Table:
| Gas Type | Common Examples | Primary Use Case | Key Characteristic |
|---|---|---|---|
| Inert Gas | Argon (Ar) | Sputtering common metals (e.g., Ti, Al) | Ideal mass, inert, cost-effective |
| Light Inert Gas | Neon (Ne) | Sputtering very light elements | Efficient momentum transfer for light atoms |
| Heavy Inert Gas | Krypton (Kr), Xenon (Xe) | Maximizing rate for heavy elements (e.g., Au, Pt) | High mass for maximum momentum transfer |
| Reactive Gas | Oxygen (O₂), Nitrogen (N₂) | Creating compound films (e.g., oxides, nitrides) | Chemically reacts with sputtered atoms |
Ready to optimize your sputtering process? The right gas is critical for achieving the desired film properties, deposition rate, and cost-efficiency. KINTEK specializes in providing high-purity lab gases and sputtering equipment tailored to your specific research and production needs. Our experts can help you select the perfect gas mixture for your target material and application.
Contact our team today to discuss your thin-film deposition challenges and discover how KINTEK's solutions can enhance your laboratory's capabilities.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- 20L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- Lab-Scale Vacuum Induction Melting Furnace
People Also Ask
- What is plasma enhanced? A Guide to Low-Temperature, High-Precision Manufacturing
- What are the benefits of PECVD? Achieve Superior Low-Temperature Thin Film Deposition
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- What are the applications of PECVD? Essential for Semiconductors, MEMS, and Solar Cells














