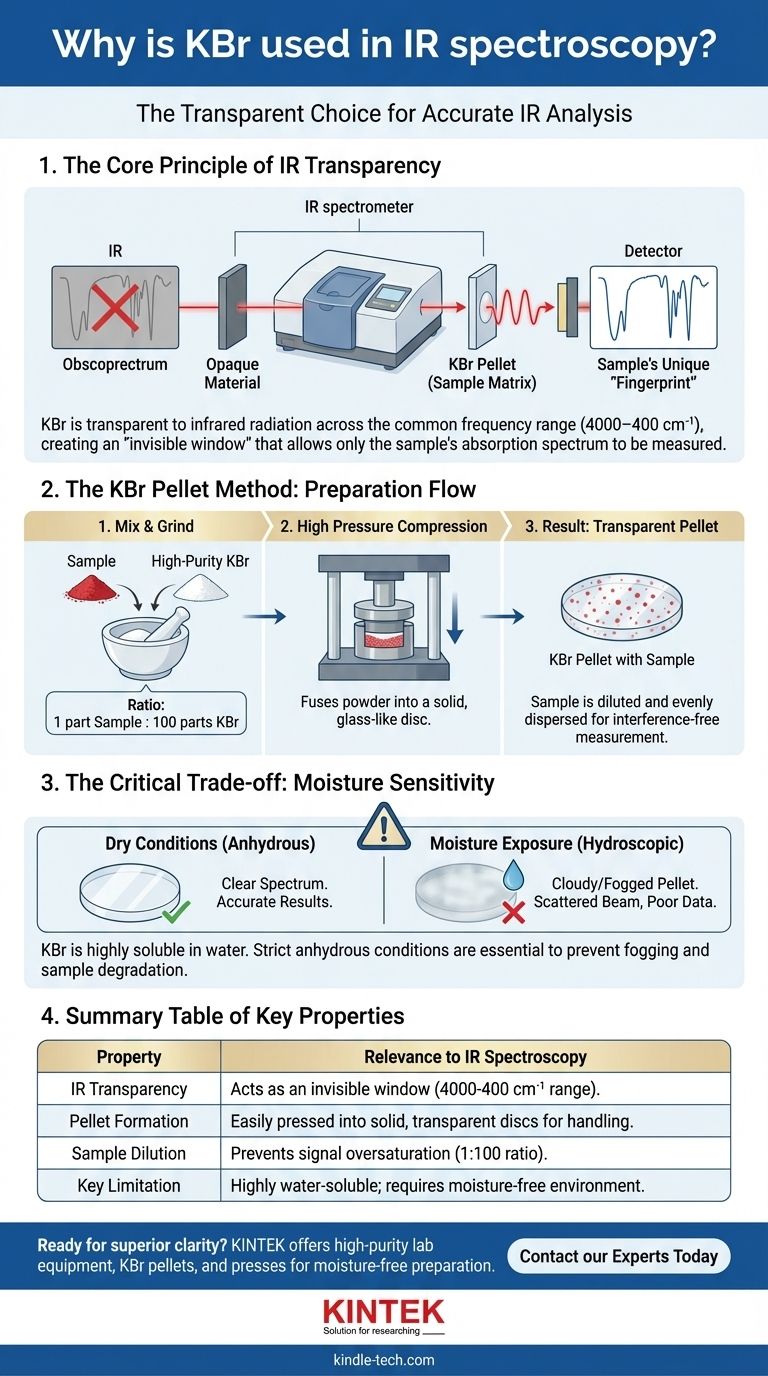
Potassium bromide (KBr) is a standard choice for IR spectroscopy because it is transparent to infrared radiation across the most commonly used frequency range. This optical transparency, combined with its ability to be pressed into a solid, glass-like pellet, makes it an ideal, non-interfering matrix for holding solid samples during analysis.
The core principle of sample preparation in IR spectroscopy is to position the material for analysis without the sample holder itself absorbing infrared light. KBr serves as this invisible window, allowing the spectrometer to measure only the sample's unique absorption spectrum, but its use demands strict, moisture-free conditions.

The Principle of IR Transparency
Why the Sample Holder Must Be "Invisible"
An IR spectrometer works by passing a beam of infrared light through a sample and measuring which frequencies are absorbed.
This absorption pattern creates a unique spectral "fingerprint" for the molecule. If the material holding the sample also absorbs IR light, its own fingerprint will overlap and obscure the signal from the sample, making the data useless.
KBr's Wide Optical Window
Alkali halide salts, most notably potassium bromide (KBr) and sodium chloride (NaCl), are exceptionally well-suited for this role.
They have simple ionic crystal structures that do not absorb light in the typical mid-infrared region (roughly 4000 to 400 cm⁻¹). They effectively create a transparent "window" through which the sample can be clearly observed.
Preparing Samples with the KBr Pellet Method
Creating a Dilute Matrix
For solid samples, the most common technique is the KBr pellet method. A very small amount of the solid sample is mixed and ground with a much larger quantity of high-purity, dry KBr powder.
A typical ratio is around 1 part sample to 100 parts KBr. This ensures the sample is diluted and dispersed evenly throughout the matrix.
The Role of High Pressure
This fine powder mixture is then placed into a pellet die and compressed with a hydraulic press at very high pressure.
The pressure causes the KBr powder to fuse, forming a thin, solid, and semi-transparent disc. The sample's particles become trapped and suspended within this solid KBr matrix.
Why This Method is Effective
This process creates a solid sample that is easy to handle and mount in the spectrometer's beam path.
The dilution prevents the sample signals from being too strong (oversaturated), and the uniform dispersion within the transparent KBr allows the IR light to pass through for a clean, high-quality measurement.
Understanding the Critical Trade-offs
High Solubility in Water
The primary disadvantage of KBr is its nature as a salt: it is highly soluble in water.
Even small amounts of atmospheric moisture can cause KBr pellets or windows to become cloudy or "fogged," scattering the IR beam and degrading the quality of the spectrum. Contact with any aqueous liquid will dissolve the pellet entirely.
The Requirement for Anhydrous Conditions
Because of this high water solubility, it is absolutely critical that the sample, the KBr powder, and any solvents used are anhydrous (completely free of water).
Failing to maintain dry conditions is the most common reason for poor results when using KBr pellets or salt plates for liquid analysis.
Alternatives for Different Samples
While KBr is standard for dry solids, other materials are used in different situations.
Liquid samples are often sandwiched between flat polished plates (or "cells") made of KBr, NaCl, or sometimes calcium fluoride (CaF₂). These plates are also highly vulnerable to moisture and must be handled with care.
Making the Right Choice for Your Sample
- If your primary focus is analyzing a dry, solid powder: The KBr pellet method is an industry-standard technique that provides excellent results, provided you maintain strictly anhydrous conditions.
- If your primary focus is analyzing a non-aqueous liquid: Sandwiching the liquid between KBr or NaCl salt plates is the conventional approach, but you must ensure your sample and any cleaning solvents are completely dry.
- If your primary focus is analyzing a sample containing water: You must avoid KBr entirely and select an insoluble window material, such as zinc selenide (ZnSe) or silver chloride (AgCl).
Ultimately, choosing KBr is a decision for excellent optical clarity in the infrared spectrum—a choice that demands meticulous handling to manage its single greatest vulnerability: moisture.
Summary Table:
| Property | Relevance to IR Spectroscopy |
|---|---|
| IR Transparency | Transparent in 4000-400 cm⁻¹ range; acts as an invisible window. |
| Pellet Formation | Can be pressed into solid, transparent discs for easy sample handling. |
| Sample Dilution | Typical 1:100 sample-to-KBr ratio prevents signal oversaturation. |
| Key Limitation | Highly soluble in water; requires strict anhydrous conditions. |
Ready to achieve superior clarity in your IR spectroscopy? KINTEK specializes in high-purity lab equipment and consumables, including KBr pellets and presses designed for moisture-free sample preparation. Our products ensure your lab achieves accurate, interference-free results. Contact our experts today to find the perfect solution for your laboratory needs!
Visual Guide
Related Products
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Grinding Mill Mortar Grinder for Sample Preparation
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- No Demolding Lab Infrared Press Mold for Laboratory Applications
People Also Ask
- Why is a laboratory hydraulic press essential for Ca3Co4O9 pelletizing? Optimize Pre-Sintering Mass Transport
- What are the advantages of using a laboratory manual hydraulic pellet press for FTIR? Enhance Your Spectral Data
- What is the role of a laboratory hydraulic press in Rare Earth Element (REE) analysis? Unlock High-Precision XRF & LIBS
- What is the necessity of using a laboratory hydraulic press for RDF TGA? Optimize Your Thermal Analysis Accuracy
- What function does a laboratory hydraulic press serve in the preparation of LLZTO ceramic electrolyte pellets?














