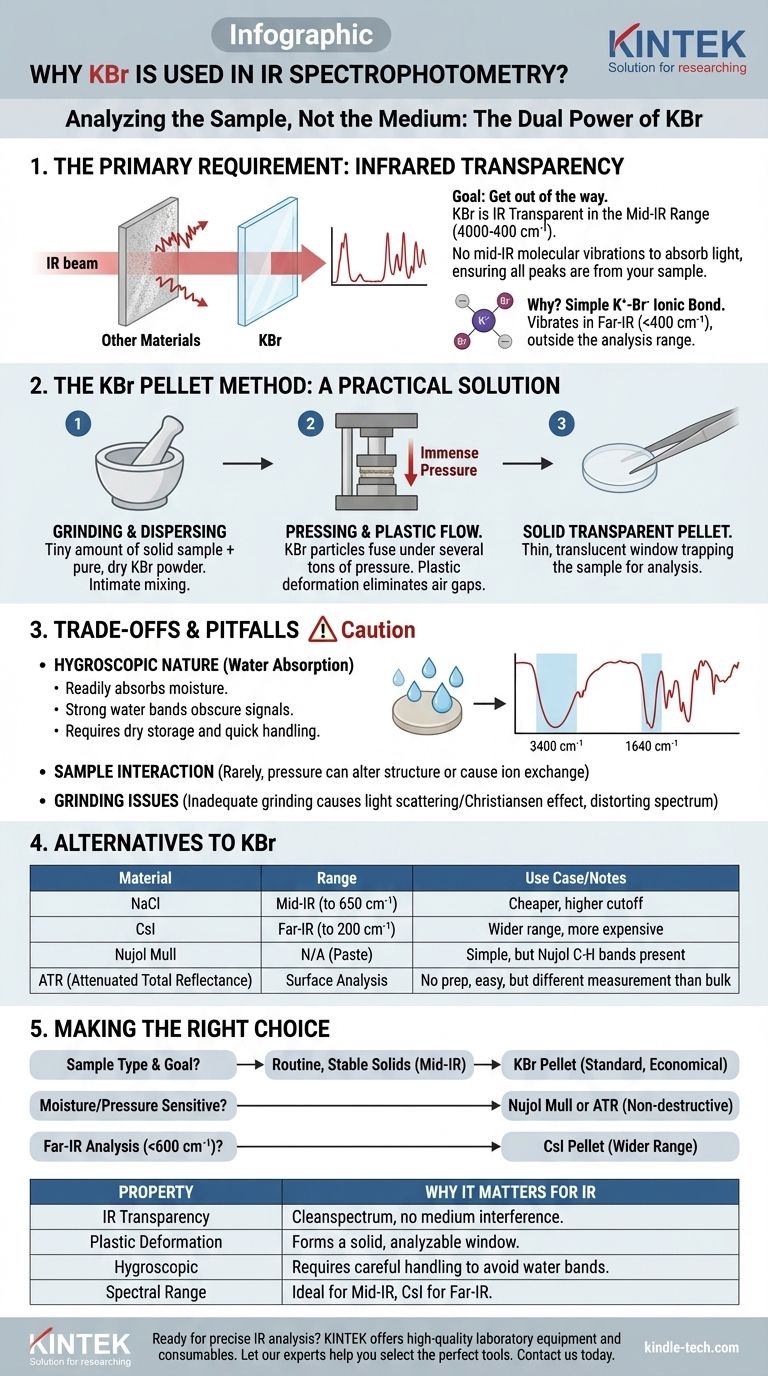
In infrared (IR) spectroscopy, the goal is to analyze the sample, not the medium holding it. For this reason, Potassium Bromide (KBr) is widely used because it is almost completely transparent to infrared radiation across the most useful frequency range. Its unique physical properties also allow it to be pressed from a powder into a solid, glass-like disk, creating a perfect window through which to analyze a solid sample.
KBr's value in IR spectroscopy isn't just that it's transparent to infrared light; it's that it combines this optical neutrality with the unique physical ability to form a solid, transparent pellet under pressure, effectively turning a difficult solid sample into an easily analyzable form.

The Primary Requirement: Infrared Transparency
The fundamental job of a matrix material in transmission IR spectroscopy is to get out of the way. KBr excels at this.
What "IR Transparent" Means
Most organic and many inorganic chemical bonds bend, stretch, and vibrate when they absorb energy from infrared light. An IR spectrometer measures which frequencies are absorbed, producing a spectrum that acts as a molecular "fingerprint."
KBr is considered IR transparent because it does not have any molecular vibrations that absorb light in the typical mid-infrared region (4000 cm⁻¹ to 400 cm⁻¹). This ensures that any absorption peaks detected in the spectrum come from the sample itself, not from the KBr holding it.
The Physics Behind the Transparency
The bond between a potassium cation (K⁺) and a bromide anion (Br⁻) is ionic. The vibrations of this simple ionic lattice are very low in energy.
This means their fundamental absorption frequency is far below 400 cm⁻¹, placing it in the "far-infrared" region, well outside the range used for most chemical structure identification.
The KBr Pellet Method: A Practical Solution
For solid samples, you cannot simply shine an IR beam through a large crystal or a pile of powder; the light would scatter or be completely blocked. KBr provides an elegant solution.
Dispersing the Sample
The KBr pellet technique involves grinding a tiny amount of the solid sample with pure, dry KBr powder. This process intimately mixes and disperses the sample molecules throughout the KBr matrix.
The Role of Pressure and Plastic Flow
This finely ground mixture is then placed in a die and subjected to immense pressure (several tons). KBr is a soft, crystalline solid that exhibits plastic deformation—under pressure, the small KBr particles fuse together, eliminating air gaps and forming a thin, translucent, or transparent solid disk.
The sample is now trapped evenly within this solid KBr window, ready for analysis.
Understanding the Trade-offs and Pitfalls
While KBr is a workhorse material, it is not without its challenges. Awareness of its limitations is critical for acquiring good data.
The Challenge of Water: KBr is Hygroscopic
The biggest drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
Water has very strong and broad IR absorption bands (around 3400 cm⁻¹ and 1640 cm⁻¹), which can easily obscure important signals from the sample. Therefore, KBr must be stored in a desiccator and handled quickly to minimize water contamination.
Potential for Sample Interaction
The high pressure used to form the pellet can sometimes alter the crystalline structure (polymorphism) of the sample. In rare cases, an ion-exchange reaction can occur between the sample and the bromide ions, creating a new substance and an invalid spectrum.
The Importance of Grinding
If the sample is not ground into particles smaller than the wavelength of the IR light, significant light scattering can occur. This phenomenon, known as the Christiansen effect, results in distorted peak shapes and a sloping baseline, making the spectrum difficult to interpret.
Are There Alternatives to KBr?
Depending on the sample and analytical goal, other materials and techniques can be used.
Other Alkali Halides
Sodium Chloride (NaCl) is cheaper than KBr and also IR transparent, but its useful range cuts off at a higher frequency (around 650 cm⁻¹). Cesium Iodide (CsI) is more expensive but offers a wider spectral window, extending down to 200 cm⁻¹, making it useful for far-IR studies.
The Nujol Mull Technique
In this method, the solid sample is ground into a paste with a mineral oil (Nujol). This mull is then spread between two salt plates (often KBr or NaCl). The primary disadvantage is that the Nujol itself has C-H absorption bands that will always be present in the spectrum.
Attenuated Total Reflectance (ATR)
Modern spectroscopy often relies on ATR, a technique that requires little to no sample preparation. A solid or liquid sample is pressed against a high-refractive-index crystal (like diamond or zinc selenide), and the IR beam analyzes the very surface of the sample. While powerful, it measures the surface differently than the bulk transmission measured with a KBr pellet.
Making the Right Choice for Your Analysis
The best sample preparation method depends entirely on your specific circumstances and analytical goals.
- If your primary focus is routine analysis of stable, non-moisture-sensitive solids: The KBr pellet method remains a highly effective and economical standard.
- If your sample is sensitive to moisture or pressure: Consider using the Nujol mull technique or a non-destructive method like ATR to avoid altering the sample.
- If you need to analyze functional groups in the low-frequency region (below 600 cm⁻¹): A KBr pellet is sufficient, but a CsI pellet is required for analysis into the far-IR range.
Understanding the properties of your matrix material is the first step toward acquiring a clean, interpretable, and accurate infrared spectrum.
Summary Table:
| Property | Why It Matters for IR Spectroscopy |
|---|---|
| IR Transparency | Does not absorb in the mid-IR range (4000-400 cm⁻¹), ensuring a clean sample spectrum. |
| Plastic Deformation | Can be pressed into a solid, transparent pellet that holds the sample for analysis. |
| Hygroscopic Nature | Absorbs water, which can interfere with the spectrum; requires careful handling. |
| Spectral Range | Ideal for mid-IR; alternatives like CsI are needed for far-IR studies. |
Ready to achieve precise and reliable IR analysis? The right lab equipment is crucial for successful sample preparation and accurate results. KINTEK specializes in providing high-quality laboratory equipment and consumables, including IR spectroscopy supplies, to meet your exact needs.
Let our experts help you select the perfect tools for your laboratory. Contact us today via our form to discuss how we can support your research and analytical goals.
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Lab Infrared Press Mold
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Grinding Mill Mortar Grinder for Sample Preparation
People Also Ask
- How do you prepare a sample for XRD? Achieve Accurate Phase Identification with Proper Technique
- What is the function of a laboratory hydraulic press in biomass analysis? Achieve Precision Pellet Preparation
- What is the working of a hydraulic process? Harness Pascal's Law for Immense Force
- How do laboratory hydraulic presses and forming molds create 3D superlattice nanocatalysts? Enhance Material Density
- Why is a uniaxial hydraulic press utilized for cold-pressing metal powders? Enhance Your Sintering Quality
- Why is my hydraulic press not holding pressure? Diagnose and Fix Common Leaks
- Why is a laboratory hydraulic press used for ASSB assembly? Achieving 392 MPa for Optimal Solid-State Battery Density
- Why KBr disc is used in IR spectroscopy? Achieve High-Resolution Analysis of Solid Samples


















