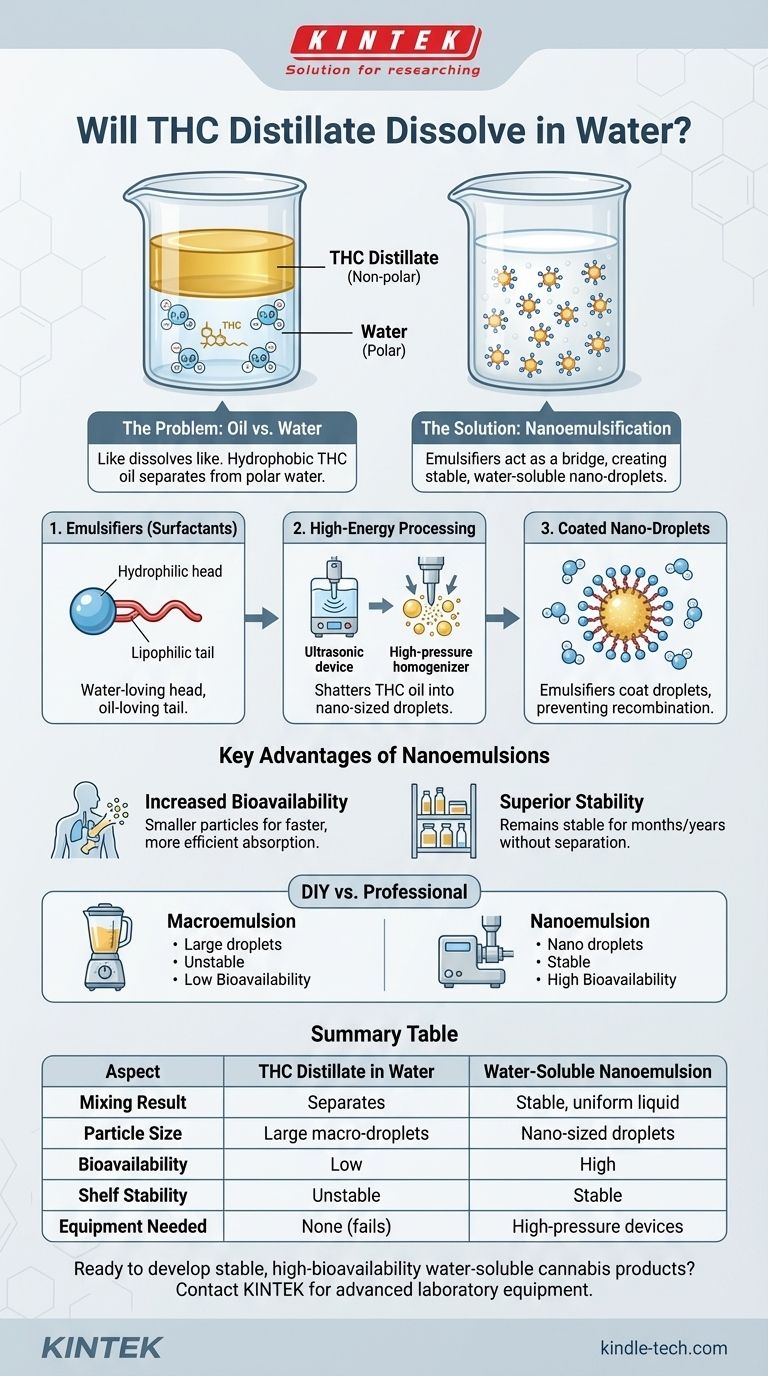
In its raw form, THC distillate will not dissolve in water. THC is an oil, and just like any other oil, it is hydrophobic, meaning it repels water. Attempting to mix the two directly will result in the distillate separating and floating on the surface, creating an inconsistent and unviable product.
The core challenge is not dissolving THC in the traditional sense, but transforming it so it can be stably suspended in water. This is achieved through a process called emulsification, which breaks the THC oil into microscopic droplets and coats them so they can mix evenly into a water-based solution.

The Fundamental Problem: Oil vs. Water
To understand why THC distillate won't dissolve in water, we need to look at the molecular level. The principle "like dissolves like" is the key.
Understanding Molecular Polarity
Water (H₂O) is a polar molecule. It has a slight positive charge on one end and a slight negative charge on the other, allowing water molecules to attract and bond with each other easily.
THC, like other fats and oils, is a non-polar molecule. It lacks the separated charges that water has, making it incompatible.
What Happens When You Try to Mix Them
When you add non-polar THC oil to polar water, the water molecules are much more attracted to each other than they are to the oil. They effectively push the oil away, causing it to clump together and separate from the water.
The Solution: Creating a Water-Soluble Emulsion
Since we cannot change the fundamental properties of THC or water, we must introduce a "bridge" to connect them. This is done by creating an emulsion.
The Role of Emulsifiers
An emulsifier (or surfactant) is a special type of molecule that has two distinct ends: a "water-loving" (hydrophilic) head and an "oil-loving" (lipophilic) tail.
This unique structure allows the emulsifier to act as a mediator. The oil-loving tail attaches to the THC oil droplet, while the water-loving head faces outward, allowing the entire particle to disperse evenly throughout the water.
Introducing Nanoemulsification
Nanoemulsification is the industry-standard technology for creating truly high-quality, water-soluble cannabis products.
This process uses high-energy methods, such as high-pressure homogenization or ultrasonic devices, to shatter the THC oil into incredibly tiny (nanometer-sized) droplets.
These nano-droplets have a massive collective surface area, which is then coated by the emulsifier. The resulting liquid is stable, consistent, and offers significantly improved performance.
Understanding the Trade-offs and Key Considerations
Creating a water-soluble THC solution is a sophisticated process with distinct advantages and complexities.
Increased Bioavailability
A primary benefit of nanoemulsions is enhanced bioavailability. The incredibly small particle size allows the body to absorb the THC more rapidly and efficiently, leading to a faster onset of effects and greater overall potency from a smaller dose.
Stability and Shelf Life
A well-formulated nanoemulsion can remain stable for months or even years without the THC oil separating from the water. Poorly made emulsions, especially those made at home, will separate quickly.
The Need for Specialized Equipment
Achieving a true nanoemulsion is not a simple DIY project. It requires industrial-grade machinery and a precise understanding of formulation science. Simple kitchen blenders cannot produce the energy required to create stable, nano-sized particles.
DIY Emulsions Have Limitations
While you can create a basic emulsion at home using an emulsifier like sunflower lecithin, the result is a macroemulsion. The oil droplets are much larger, less stable, and offer lower bioavailability. This method may be suitable for immediate consumption but not for a shelf-stable product.
Making the Right Choice for Your Goal
To properly infuse THC into a water-based product, you must align your method with your intended outcome.
- If your primary focus is a professional, shelf-stable beverage: You must use nanoemulsification technology, which requires investing in specialized equipment and formulations.
- If your primary focus is a simple home-infusion for immediate use: A basic macroemulsion using a carrier oil, lecithin, and a blender can work, but expect potential separation and inconsistent effects.
- If your primary focus is ease and consistency without water: Infusing THC distillate directly into fats like butter or coconut oil for use in traditional edibles remains the most straightforward method.
Understanding the science of solubility is the key to creating effective, consistent, and predictable water-based cannabis products.
Summary Table:
| Aspect | THC Distillate in Water | Water-Soluble Nanoemulsion |
|---|---|---|
| Mixing Result | Separates (oil & water) | Stable, uniform liquid |
| Particle Size | Large macro-droplets | Nano-sized droplets |
| Bioavailability | Low | High (faster onset) |
| Shelf Stability | Unstable, separates quickly | Stable for months/years |
| Equipment Needed | None (direct mix fails) | High-pressure homogenizers / ultrasonic devices |
Ready to develop stable, high-bioavailability water-soluble cannabis products?
At KINTEK, we specialize in the advanced laboratory equipment and consumables required for precise nanoemulsification. Our high-pressure homogenizers and ultrasonic processors are engineered to help you create consistent, shelf-stable formulations that maximize potency and consumer experience.
Let's transform your product development process. Contact our experts today to discuss your specific lab needs and how we can support your innovation in cannabis infusion technology.
Visual Guide
Related Products
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Vacuum Cold Trap Chiller Indirect Cold Trap Chiller
- Single Punch Electric Tablet Press Machine TDP Tablet Punching Machine
- 80L Chilling Circulator Cooling Water Circulator for Water Bath Cooling and Low Temperature Constant Temperature Reaction Bath
People Also Ask
- How do Ultra-Low Temperature freezers contribute to public health? Preserving Vaccines and Research for a Healthier World
- How do industrial-grade constant temperature drying ovens ensure GO anti-corrosion coating performance?
- What are the advantages of plastic pyrolysis process? Unlock Value from Waste Plastic
- What are the types of pyrolysis temperature? A Guide to Low, Medium, and High-Temperature Pyrolysis
- What is the process of pyrolysis in biomass energy? A Guide to Converting Biomass into Biofuel, Biochar, and Syngas
- How do ultrasonic homogenizers or cell disruptors enhance the digestion efficiency of substrates in dark fermentation? Boost Bio-Availability
- What are the 3 types of biomass energy sources? Unlock the Potential of Organic Materials
- What is the temperature range for heat treatment of steel? Mastering the Critical Temperatures for Desired Properties


















