In the laboratory, we are often obsessed with the chemistry. We meticulously control the electrolyte concentration, the purity of the catalyst, and the voltage applied.
Yet, we frequently overlook the physical constraints that contain that chemistry.
There is a specific danger in experimental science: assuming that because you measured something, the number is real. But a number without context is just noise.
In electrochemical cells, the difference between noise and signal often comes down to a single physical dimension: the reaction area.
The Standardization of Chaos
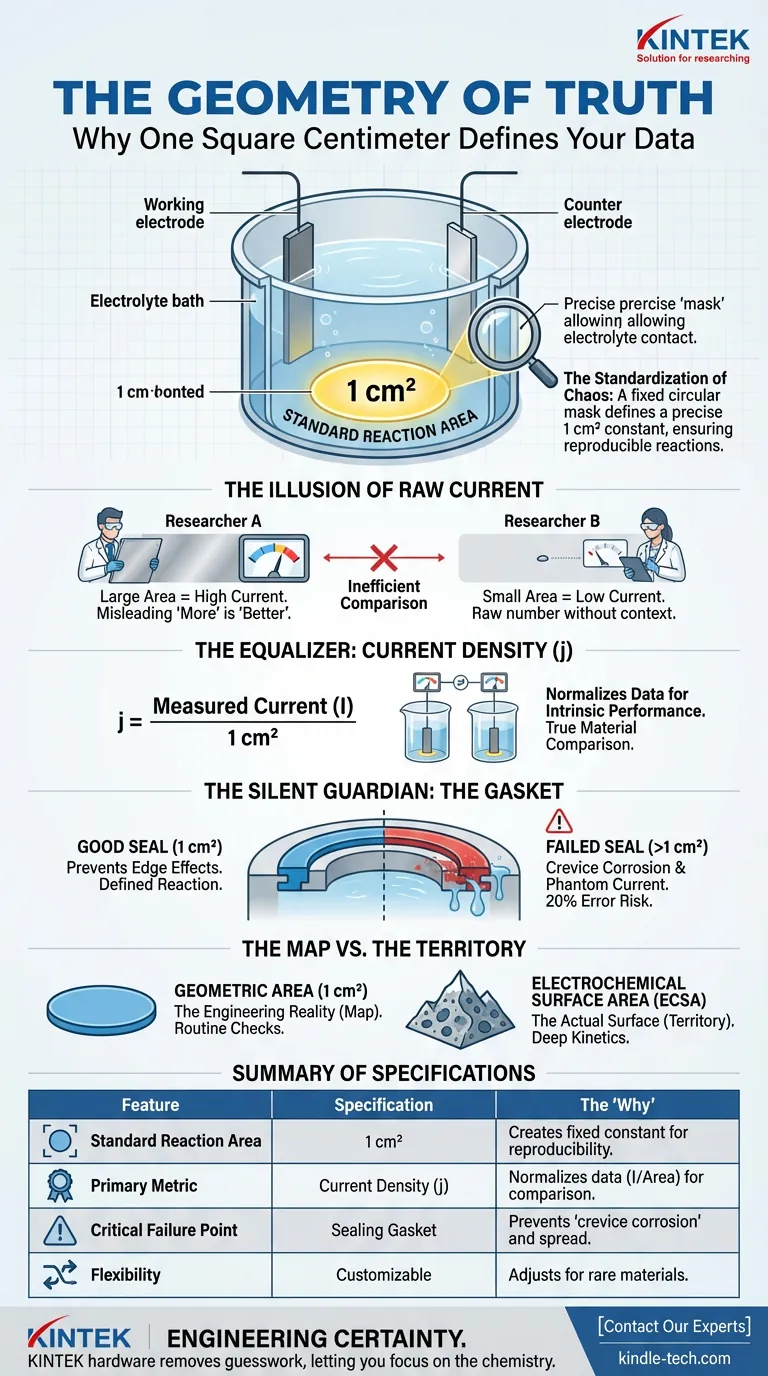
The electrolytic cell in question features a fixed circular hole at the bottom. This is not an arbitrary design choice. It is a precise engineering control.
This hole defines a standard reaction area of 1 square centimeter (1 cm²).
Why 1 cm²? Because in a world of infinite variables, you need a constant. This defined opening acts as a mask, allowing the electrolyte to touch only a specific, known portion of your working electrode.
Without this mask, your reaction spreads. It becomes undefined. And an undefined reaction cannot be repeated.
The Illusion of Raw Current
Imagine two researchers testing the same catalyst.
- Researcher A uses a massive sheet of metal.
- Researcher B uses a tiny speck.
Researcher A will measure a higher current every time. Does that mean their catalyst is better? No. It just means they used more of it.
This is where the psychology of numbers tricks us. "More" feels like "better," but in electrochemistry, efficiency is king.
The Equalizer: Current Density
To compare apples to apples, we must move from raw current (Amps) to Current Density (Amps/cm²).
The math is simple, but the implication is profound:
Current Density (j) = Measured Current (I) / 1 cm²
By locking the denominator (the area) to exactly 1 cm², the cell forces the data to reflect the intrinsic performance of the material, not the size of the sample cut.
The Silent Guardian: The Gasket
The engineering challenge isn't just cutting a 1 cm² hole. It is sealing it.
Between the cell body and your sample lies a gasket. This humble component is the only thing stopping the electrolyte from creeping sideways.
If that seal fails, two things happen:
- Edge Effects: The reaction density spikes at the perimeter, skewing data.
- Crevice Corrosion: Chemistry happens in the hidden gaps, adding "phantom current" to your reading.
A worn gasket transforms a 1 cm² experiment into a 1.2 cm² guess. In precision kinetics, that 20% error is the difference between a breakthrough and a failed hypothesis.
The Map vs. The Territory
There is a final layer of complexity—the difference between the map (Geometric Area) and the territory (Electrochemical Surface Area).
The Geometric Area is the 1 cm² flat circle defined by the hardware. It is the standard map we use for comparison.
However, if you zoom in, your electrode might be a porous sponge or a rough mountain range. The Electrochemical Surface Area (ECSA) is the actual surface area of those mountains.
- For routine checks: Use the 1 cm² Geometric Area. It represents the engineering reality.
- For deep kinetics: You must calculate ECSA, but you still need the Geometric Area as your baseline control.
Summary of Specifications
The following table breaks down the critical relationship between the hardware and the data:
| Feature | Specification | The "Why" |
|---|---|---|
| Standard Reaction Area | 1 cm² | Creates a fixed geometric constant for reproducibility. |
| Primary Metric | Current Density | Normalizes data (I / Area) to allow material comparison. |
| Critical Failure Point | Sealing Gasket | Prevents "crevice corrosion" and undefined reaction spread. |
| Flexibility | Customizable | Allows adjustment for rare or low-conductivity materials. |
Engineering Certainty
At KINTEK, we understand that great science is built on the back of reliable hardware.
Our electrolytic cells are designed with a precisely machined 1 cm² reaction area to remove the guesswork from your calculations. We engineer the constraints so you can focus on the chemistry.
Don't let physical variables become experimental errors.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Measuring Cylinder 10/50/100ml
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Laboratory manual slicer
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
Related Articles
- Manual Pellet Press: A Comprehensive Guide to Efficient Lab Pelletizing
- Mastering Handheld Coating Thickness Gauges: A Comprehensive Guide for Industrial and Automotive Applications
- Understanding and Selecting the Right Microplates for Laboratory Applications
- Comprehensive Guide to Integrated Manual Heated Lab Pellet Presses
- Hazards and Safety Precautions of Laboratory Pressure Vessels





