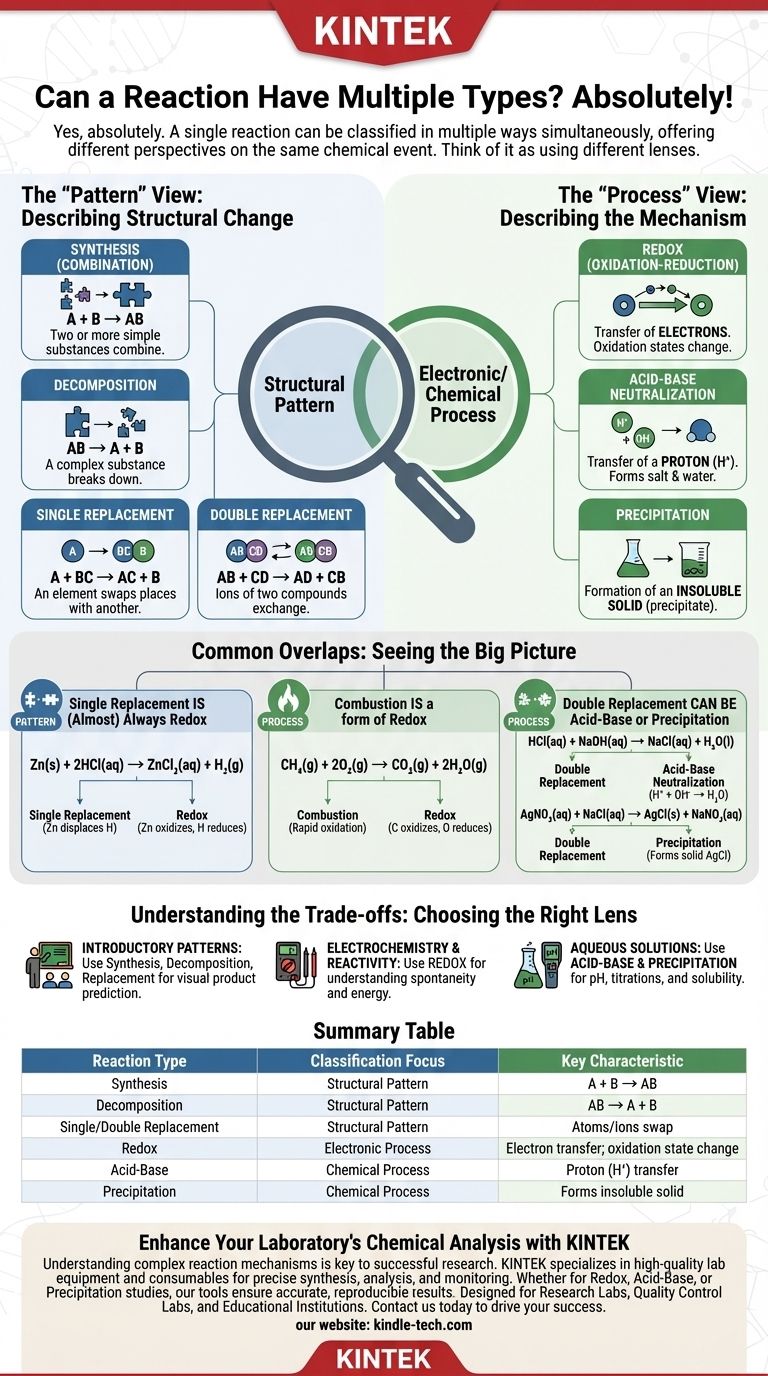
Yes, absolutely. A single chemical reaction can often be classified as multiple reaction types simultaneously. This is a common point of confusion because introductory chemistry often presents reaction types as mutually exclusive categories, but a more advanced understanding reveals they are simply different lenses for analyzing the same chemical event.
The key is to understand that some reaction types describe the structural pattern of how atoms rearrange (like synthesis or decomposition), while others describe the underlying electronic or chemical process (like redox or acid-base). A single reaction can exhibit both a specific pattern and a specific process.

Why Simple Labels Aren't Enough
The way we classify reactions depends on what we want to understand about them. The simple categories learned first are useful for recognizing visual patterns, but they don't always explain the fundamental chemical change.
The "Pattern" View: Describing Structural Change
Early in your chemistry education, you learn to recognize reactions by how the reactants form products. These categories describe the overall "shape" of the transformation.
The main pattern-based types are:
- Synthesis (or Combination): Two or more simple substances combine to form a more complex product (
A + B → AB). - Decomposition: A complex substance breaks down into simpler ones (
AB → A + B). - Single Replacement: An element swaps places with another in a compound (
A + BC → AC + B). - Double Replacement: The ions of two compounds exchange places to form two new compounds (
AB + CD → AD + CB).
These labels are excellent for quickly predicting products based on a familiar pattern.
The "Process" View: Describing the Mechanism
More sophisticated classifications describe how the transformation occurs at a molecular or electronic level. They focus on the fundamental process driving the reaction.
The main process-based types include:
- Redox (Oxidation-Reduction): This type involves the transfer of electrons. The oxidation states of one or more elements change during the reaction.
- Acid-Base Neutralization: An acid and a base react, typically involving the transfer of a proton (H⁺ ion) to form a salt and water.
- Precipitation: Two aqueous solutions are mixed, and an insoluble solid (a precipitate) is formed.
These labels are essential for understanding the underlying driving forces of a reaction.
Common Overlaps You Will Encounter
Seeing how these categories overlap is the best way to solidify your understanding. Most reactions you encounter will have more than one valid label.
Single Replacement is (Almost) Always Redox
Consider the reaction of zinc metal with hydrochloric acid:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
This is a classic single replacement reaction because zinc displaces hydrogen. However, it is also a redox reaction because the oxidation states change: zinc is oxidized (0 to +2) and hydrogen is reduced (+1 to 0).
Combustion is a form of Redox
The combustion of methane is a good example:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
While we call this combustion, it is also fundamentally a redox reaction. Carbon is oxidized (-4 to +4) and oxygen is reduced (0 to -2).
Double Replacement can be Acid-Base or Precipitation
Consider the neutralization of hydrochloric acid with sodium hydroxide:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This is a double replacement reaction; Na⁺ and H⁺ swap places. More importantly, it is the definitive example of an acid-base neutralization.
Similarly, mixing silver nitrate and sodium chloride:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
This fits the double replacement pattern, but its most notable feature is the formation of a solid, making it a precipitation reaction.
Understanding the Trade-offs: The Purpose of Classification
Choosing a label is not about finding the one "correct" answer. It's about using the most descriptive and useful label for your specific goal.
The Limitation of Simple Patterns
Classifying a reaction as "single replacement" tells you what happened to the atoms, but it doesn't explain why it happened. It doesn't explain why zinc reacts with HCl but copper does not.
The Power of Mechanistic Labels
Identifying that same reaction as "redox" provides much deeper insight. It allows you to use concepts like activity series or standard electrode potentials to predict whether the reaction will occur spontaneously and to quantify the energy involved. The redox label explains the "why."
Making the Right Choice for Your Goal
Use the classification that best serves your purpose. The goal is not to find a single, exclusive label, but to apply the most relevant one.
- If your primary focus is recognizing basic patterns in an introductory course: Stick to synthesis, decomposition, and single/double replacement to predict products visually.
- If your primary focus is electrochemistry or predicting reactivity: The redox classification is the most important lens to use.
- If your primary focus is working with aqueous solutions, pH, and titrations: Classifying reactions as acid-base or precipitation will be the most useful approach.
Ultimately, using the right label depends on the specific question you are trying to answer about the chemical transformation.
Summary Table:
| Reaction Type | Classification Focus | Key Characteristic |
|---|---|---|
| Synthesis | Structural Pattern | Two or more reactants combine into one product (A + B → AB) |
| Decomposition | Structural Pattern | One compound breaks down into simpler substances (AB → A + B) |
| Single/Double Replacement | Structural Pattern | Atoms or ions swap between compounds |
| Redox | Electronic Process | Involves transfer of electrons; oxidation states change |
| Acid-Base | Chemical Process | Involves proton (H⁺) transfer |
| Precipitation | Chemical Process | Formation of an insoluble solid from aqueous solutions |
Enhance Your Laboratory's Chemical Analysis with KINTEK
Understanding complex reaction mechanisms is key to successful research and development. At KINTEK, we specialize in providing high-quality lab equipment and consumables that support precise chemical synthesis, analysis, and process monitoring. Whether you're working on redox reactions, acid-base titrations, or precipitation studies, our reliable tools help you achieve accurate and reproducible results.
Our products are designed for:
- Research Laboratories requiring precise temperature control for synthesis and decomposition reactions.
- Quality Control Labs needing consistent equipment for acid-base and precipitation analyses.
- Educational Institutions seeking durable and easy-to-use apparatus for teaching fundamental and advanced reaction types.
Let KINTEK be your trusted partner in advancing your laboratory's capabilities. Contact us today to discuss your specific needs and discover how our solutions can drive your success.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
- Laboratory Hybrid Tissue Grinding Mill
People Also Ask
- Why is a vacuum distillation system necessary during the synthesis of rosin allyl esters? Protect Product Integrity
- How is a vacuum drying oven utilized in the study of sludge? Preserving Integrity for Precision Analysis
- What is the application of RF and DC sputtering? Choosing the Right Technique for Your Material
- What are the applications of compressed air systems? Powering Industry from Manufacturing to Pharma
- What are the applications of XRD and XRF? Unlock the Difference Between Elemental and Structural Analysis
- What are the forensic applications of XRF? Uncover the Elemental Fingerprint of Evidence
- How does a precision heating system influence the coating quality of soft magnetic composite materials? Expert Insights
- How do Ultra-Low Temperature freezers contribute to public health? Preserving Vaccines and Research for a Healthier World






