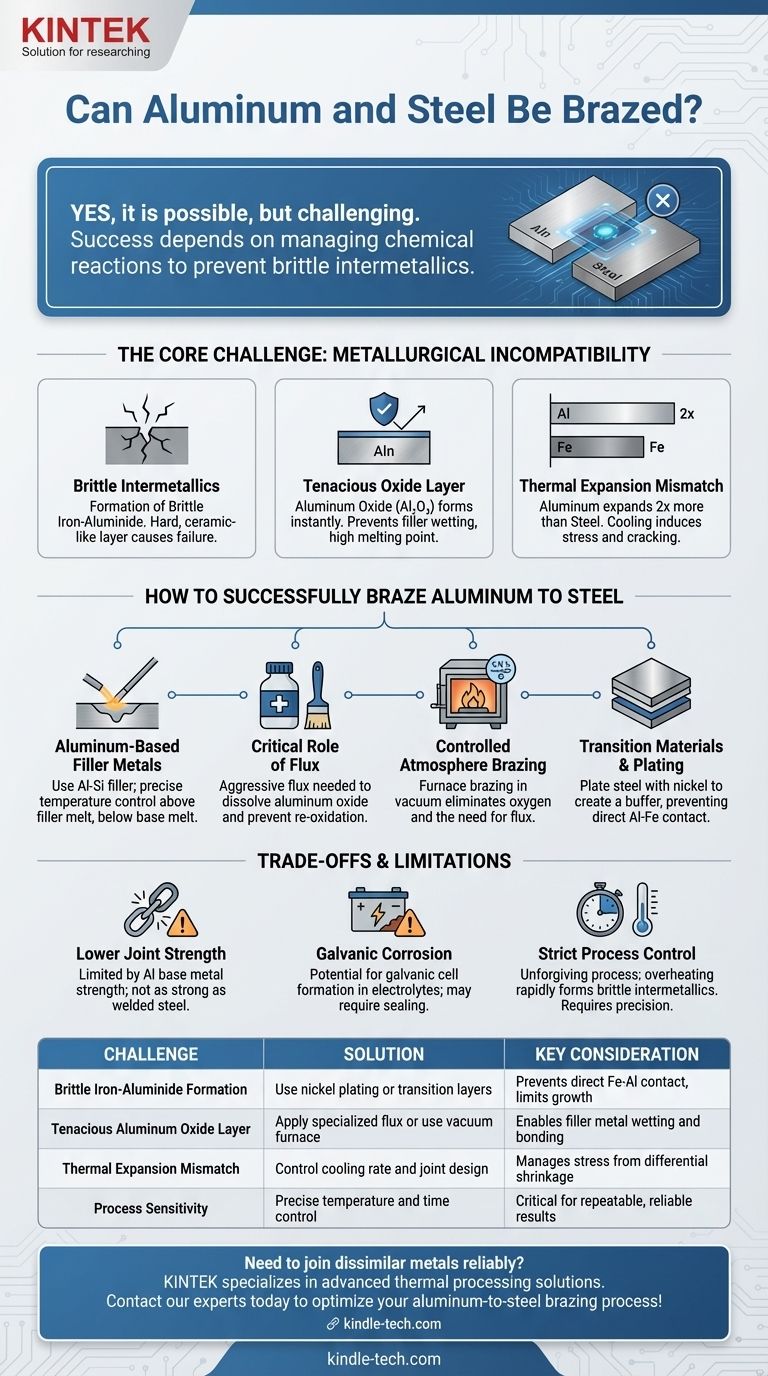
Yes, it is possible to braze aluminum to steel, but it is a challenging process that demands specialized techniques to overcome fundamental metallurgical incompatibilities. Unlike brazing similar metals, simply applying heat and a standard filler alloy will not work and is almost certain to result in a failed joint.
The core challenge in brazing aluminum to steel is not the process itself, but managing the chemical reaction at the joint. Success depends entirely on preventing the formation of brittle iron-aluminide intermetallic compounds, which requires precise control over temperature, time, and materials.
The Core Challenge: Metallurgical Incompatibility
To understand why this joint is difficult, you must first understand the conflicting properties of the two base metals. The problems go far beyond simply melting a filler metal between them.
The Problem of Brittle Intermetallics
When aluminum and iron (the primary component of steel) are heated in direct contact, they react to form iron-aluminide intermetallic compounds. These compounds are extremely hard and brittle, like a thin layer of ceramic at the joint interface.
A joint containing these brittle compounds will have very poor mechanical properties and will likely crack under minimal stress or vibration. The entire goal of a successful aluminum-to-steel brazing process is to limit or prevent the growth of this layer.
The Tenacious Oxide Layer
Aluminum instantly forms a tough, transparent layer of aluminum oxide (Al₂O₃) upon exposure to air. This oxide has a very high melting point (around 2072°C or 3762°F), which is far above the melting point of aluminum itself.
Before the brazing filler metal can "wet" and bond to the aluminum surface, this oxide layer must be chemically removed using an aggressive flux or physically prevented from forming in a vacuum furnace.
The Mismatch in Thermal Expansion
Aluminum expands and contracts with temperature changes at roughly twice the rate of steel. During the cooling phase after brazing, the aluminum will try to shrink much more than the steel.
This differential shrinkage induces significant stress at the joint, which can cause distortion, loss of tolerance, or even immediate cracking, especially if a brittle intermetallic layer is present.
How to Successfully Braze Aluminum to Steel
Overcoming these challenges requires a carefully controlled process that addresses each issue directly. There is very little room for error.
Using Aluminum-Based Filler Metals
The most common method uses an aluminum-silicon (Al-Si) filler metal. The brazing temperature for these alloys is carefully chosen to be above the filler's melting point but safely below the melting point of the aluminum base metal.
The Critical Role of Flux
For torch or induction brazing, a highly active flux is non-negotiable. This is not the same flux used for copper or steel. It must be specifically formulated to aggressively dissolve the resilient aluminum oxide layer and protect the joint from re-oxidizing during the heating cycle.
Controlled Atmosphere Brazing
For high-volume production, furnace brazing in a controlled atmosphere is the preferred method. This is often done in a vacuum, which removes the oxygen and prevents oxides from forming in the first place, eliminating the need for corrosive flux.
Transition Materials and Plating
A highly effective industrial technique involves creating a buffer between the two metals. The steel part can be "buttered" or plated with a compatible material, such as nickel. The aluminum is then brazed to this intermediate layer, preventing direct contact between the iron and aluminum and stopping the formation of brittle intermetallics.
Understanding the Trade-offs and Limitations
Even when executed perfectly, a brazed aluminum-to-steel joint has inherent compromises that you must consider for your application.
Lower Joint Strength
The resulting joint will be limited by the strength of the aluminum base metal and the filler alloy. It will not have the strength of a welded steel assembly. The design must accommodate these lower strength characteristics.
Potential for Galvanic Corrosion
Joining two dissimilar metals like aluminum and steel creates a galvanic cell. In the presence of an electrolyte (like moisture), the more active metal (aluminum) will preferentially corrode. The finished joint may require sealing or coating to prevent long-term environmental degradation.
Strict Process Control
This is not a forgiving process. Overheating the joint, even for a few seconds, can dramatically accelerate the growth of the brittle intermetallic layer, ruining the joint's integrity. Precise temperature and time control are essential for repeatable success.
Making the Right Choice for Your Application
Choosing the correct method depends entirely on your project's goals, volume, and required reliability.
- If your primary focus is prototyping or a one-off assembly: Using a specialized, flux-cored aluminum brazing rod is feasible, but requires significant practice to master temperature control.
- If your primary focus is high-volume production and reliability: A controlled furnace brazing process, often involving plating the steel component, is the only commercially viable path.
- If your primary focus is maximum strength and durability: Re-evaluate if brazing is the right method; mechanical fasteners or specialized structural adhesives might be more robust alternatives.
Successfully joining aluminum and steel with brazing requires treating it not as a simple joining task, but as a precise metallurgical process.
Summary Table:
| Challenge | Solution | Key Consideration |
|---|---|---|
| Brittle Iron-Aluminide Formation | Use nickel plating or transition layers | Prevents direct Fe-Al contact, limits intermetallic growth |
| Tenacious Aluminum Oxide Layer | Apply specialized flux or use vacuum furnace | Enables filler metal wetting and bonding |
| Thermal Expansion Mismatch | Control cooling rate and joint design | Manages stress from differential shrinkage |
| Process Sensitivity | Precise temperature and time control | Critical for repeatable, reliable results |
Need to join dissimilar metals reliably? At KINTEK, we specialize in advanced thermal processing solutions for challenging material combinations. Our expertise in controlled atmosphere brazing and specialized lab equipment can help you achieve strong, durable aluminum-to-steel joints for your R&D or production needs. Let's discuss your application — contact our experts today to optimize your brazing process!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What role does a high-temperature vacuum furnace play in the synthesis of (V1/2Mo1/2)2AlC MAX phase materials? (Synthesis Guide)
- What is the industrial brazing process? A Guide to Strong, Permanent Metal Joining
- What is classification of heating furnace? A Guide to Selecting the Right Industrial Furnace
- What problems can result from heat treating metal? Avoid Cracking, Warping, and Soft Spots
- How does a vertical cylindrical furnace facilitate the titanium electrolysis process? Precision Heat & Shielding
- Why Use a Vacuum Reactor to Dry OTMO? Ensure High-Purity Epoxy Urethane Oligomer Synthesis
- What is carburizing in heat treatment process? Create a Hard Surface with a Tough Core
- What is the difference between liquid phase sintering and solid phase sintering? Achieve Optimal Material Density



















