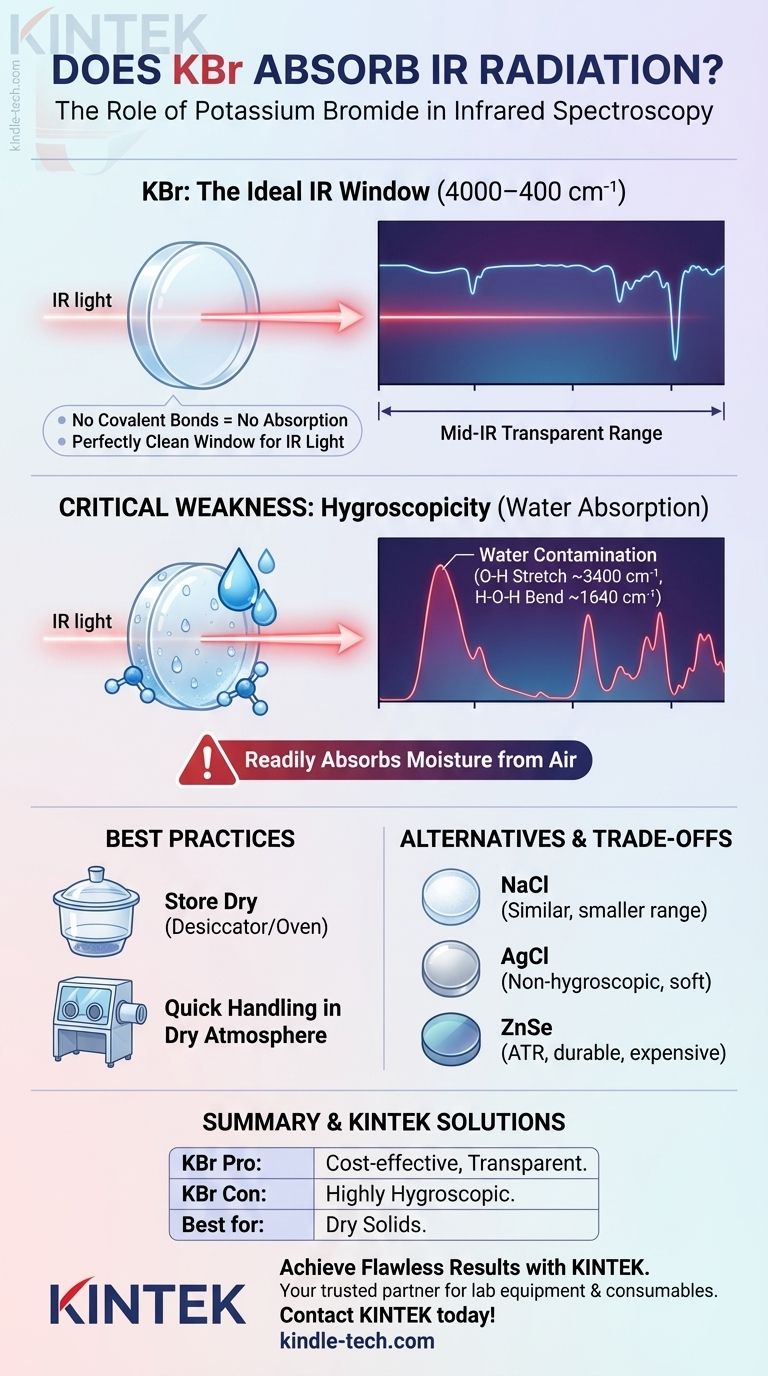
For most practical purposes in infrared spectroscopy, potassium bromide (KBr) does not absorb IR radiation in the mid-infrared region. Its atomic structure lacks the covalent bonds that vibrate and absorb energy in this specific range. This optical transparency is precisely why it is one of the most common materials used for making sample windows and pellets for analysis.
The core function of KBr in IR spectroscopy is not to be analyzed, but to serve as an invisible matrix or window. Its value lies in its lack of absorption in the key analytical region, allowing the spectrometer to measure the sample's spectrum without interference. However, this property is severely compromised by its tendency to absorb water.

The Role of KBr in IR Spectroscopy
To understand KBr, you must see it as a tool, not a sample. Its job is to hold your compound of interest in the path of the IR beam without adding any spectral information of its own.
A Window to the Molecular World
Think of KBr as a perfectly clean, clear window for infrared light. Just as you look through a glass window to see the view outside, an IR spectrometer looks through the KBr to see the molecular vibrations of your sample.
The goal is to obtain a spectrum of only your analyte. Using an IR-transparent material like KBr ensures that the peaks you observe belong to your compound, not the material holding it.
The Ideal Wavenumber Range
KBr is exceptionally transparent across the most useful portion of the infrared spectrum, typically from 4000 cm⁻¹ down to about 400 cm⁻¹. This range covers the vast majority of fundamental molecular vibrations—stretches, bends, and twists—that are used to identify functional groups and characterize a compound's structure.
Why Simple Ionic Solids are Different
The compounds we typically analyze with IR spectroscopy (e.g., organic molecules, polymers) are held together by covalent bonds. These bonds vibrate at specific frequencies when exposed to IR radiation, creating an absorption spectrum.
KBr, in contrast, is a simple ionic salt. The vibration of its crystal lattice occurs at a much lower frequency, falling into the far-infrared region (below 400 cm⁻¹). Therefore, in the mid-IR range, it is effectively invisible.
Understanding the Trade-offs: The Critical Weakness of KBr
While KBr is an excellent material in principle, it has one significant practical drawback that every analyst must manage: its interaction with water.
The Problem with Water (Hygroscopicity)
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. This is its single greatest weakness in a laboratory setting.
Even brief exposure to humid air can cause enough water to adsorb onto the KBr powder or pellet surface to become a significant contaminant in your analysis.
Water's Impact on Your Spectrum
When water is present in your KBr, it will dominate your spectrum with its own strong absorption peaks. You will typically see:
- A very broad, strong peak around 3400 cm⁻¹ (from O-H stretching).
- A medium-intensity peak around 1640 cm⁻¹ (from H-O-H bending).
These water peaks can easily obscure important sample signals, such as N-H or O-H stretches from your actual compound, leading to incorrect interpretations.
Best Practices for Handling KBr
To get a clean spectrum, you must handle KBr with care. Always use spectroscopy-grade KBr and store it in a desiccator or a drying oven (at ~110 °C) to keep it anhydrous. When preparing a pellet, work quickly and consider using a glove box with a dry atmosphere if possible.
KBr vs. Other IR Materials
KBr is not the only choice for IR analysis. Understanding the alternatives helps you select the right tool for the job.
Sodium Chloride (NaCl)
Like KBr, NaCl is an inexpensive and hygroscopic ionic salt. However, its useful range is smaller, as it begins to absorb IR radiation around 650 cm⁻¹. This makes it unsuitable if you need information in the lower-frequency "fingerprint" region.
Silver Chloride (AgCl)
AgCl is soft, pliable, and, most importantly, not hygroscopic. This makes it useful for analyzing aqueous samples. However, it is sensitive to light, more expensive than KBr, and will corrode metal parts, so it must be handled carefully.
Zinc Selenide (ZnSe)
ZnSe is a hard, durable, and non-hygroscopic material with a wide transparent range. It is an excellent but expensive material, commonly used as the internal reflection crystal in ATR-FTIR accessories. Its durability and water-insolubility make it ideal for routine, high-throughput analysis of liquids and solids.
Making the Right Choice for Your Goal
Selecting the correct IR material depends entirely on your sample, budget, and analytical needs.
- If your primary focus is routine analysis of dry, solid samples: KBr pellets remain the most cost-effective and established method, provided you handle the material correctly to avoid moisture.
- If your primary focus is analyzing aqueous solutions or wet samples: Avoid KBr entirely and choose a water-insoluble material like AgCl for transmission or, more commonly, use an ATR accessory with a ZnSe or diamond crystal.
- If your primary focus is durability and minimal sample prep: An ATR-FTIR setup with a ZnSe or diamond crystal is the superior modern choice, eliminating the need for pellets and concerns about hygroscopicity.
Understanding the properties of your matrix material is the first step toward achieving a clean and accurate spectrum.
Summary Table:
| Property | KBr for IR Spectroscopy |
|---|---|
| IR Transparency | Excellent in mid-IR range (4000–400 cm⁻¹) |
| Primary Use | Sample pellets and windows for transmission FTIR |
| Key Advantage | Cost-effective, minimal spectral interference |
| Critical Weakness | Highly hygroscopic (absorbs atmospheric moisture) |
| Handling Requirement | Must be stored in a desiccator and kept dry during use |
| Best For | Routine analysis of dry, solid samples |
Achieve Flawless FTIR Results with the Right Equipment
Understanding the properties of your sample preparation materials is crucial for accurate spectral analysis. Whether you're working with traditional KBr pellets or modern ATR accessories, having the right, high-quality lab equipment is the foundation of reliable data.
KINTEK is your trusted partner for all your laboratory needs. We specialize in providing robust and precise lab equipment and consumables, including materials for FTIR spectroscopy. Our expertise ensures you have the reliable tools necessary to handle sensitive materials like KBr correctly and obtain clean, interpretable spectra every time.
Ready to enhance your analytical capabilities? Let our experts help you select the perfect equipment for your specific application.
Contact KINTEK today to discuss your lab's requirements and discover how our solutions can drive your research forward!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- kbr pellet press 2t
- Lab Infrared Press Mold
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
People Also Ask
- What type of oven is used in the lab? Precision Heating for Scientific Accuracy
- What is ferronickel used for? The Essential Alloy for Stainless Steel Production
- Why is a laboratory magnetic stirrer required for benzoic acid esters? Boost Reaction Speed & Yield with High RPM
- Can you separate the solid and liquid in a solution by filtering? No, and Here's Why
- What are the results of sintering? From Powder to High-Strength Solid Parts
- At what temperature does graphite thermal decompose? The Critical Role of Atmosphere
- What are the processes that need to be done after completing the sintering stage? Master the Critical Post-Sintering Steps
- What is heat treatment in manufacturing process? Transform Material Properties for Superior Performance



















