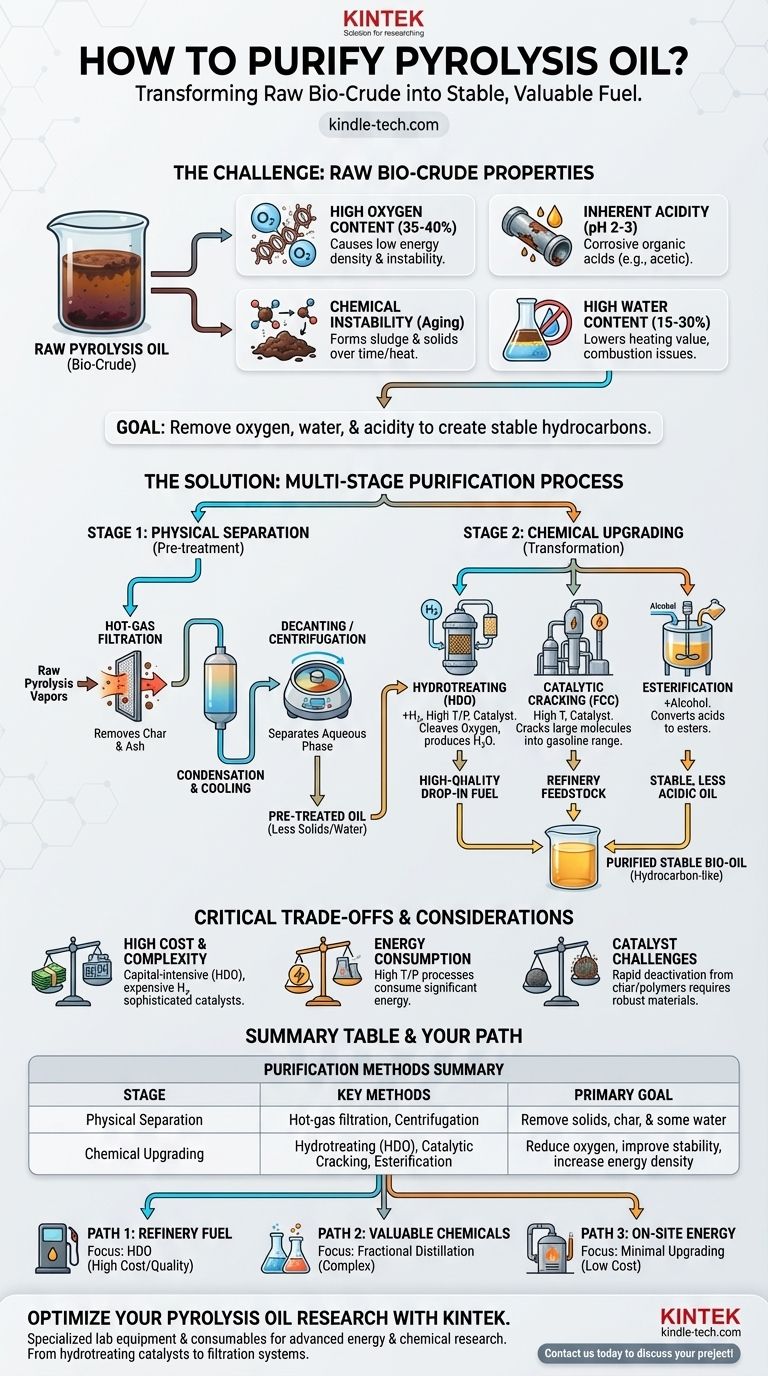
Purification of pyrolysis oil is a multi-stage process involving physical separation and chemical upgrading to improve its stability, energy density, and usability. The raw bio-crude is highly acidic, unstable, and contains significant amounts of water and oxygen, making it unsuitable for direct use as a fuel or refinery feedstock. The goal is to transform it into a more conventional, hydrocarbon-like liquid through methods like filtration, distillation, and hydrotreating.
Raw pyrolysis oil is fundamentally different from conventional crude oil, and "purification" is less about simple cleaning and more about intensive chemical upgrading. The primary challenge is removing oxygen, which requires significant investment in processes that can be technically complex and costly, especially at a smaller scale.

Why Raw Pyyrolysis Oil Requires Upgrading
Before discussing how to purify it, we must understand why the raw product, often called bio-crude or bio-oil, is so problematic. Its unique chemical composition presents several major challenges that prevent its direct use.
The Problem of High Oxygen Content
Raw pyrolysis oil contains a high concentration of oxygen (35-40% by weight), locked within molecules like acids, aldehydes, ketones, and phenols. This oxygen is the root cause of its low energy density, corrosiveness, and chemical instability compared to conventional hydrocarbon fuels, which have almost no oxygen.
Inherent Acidity and Corrosiveness
The presence of organic acids, primarily acetic and formic acid, makes the oil highly acidic (pH of 2-3). This level of acidity makes it corrosive to standard pipes, tanks, and engine components, requiring expensive specialized materials for handling and storage.
Chemical Instability and Aging
Pyrolysis oil is thermally unstable. Over time, or when heated, its reactive molecules (like aldehydes and phenols) polymerize, forming thick sludge and solids. This "aging" process increases the oil's viscosity, making it difficult to pump and use.
High Water Content
The oil is also immiscible with hydrocarbon fuels due to its high water content (15-30%), which is produced during the pyrolysis reaction and is also present from the original biomass. This water further lowers the oil's heating value and can cause issues in combustion systems.
Key Purification and Upgrading Methods
Purification is a step-by-step process that moves from simple physical separation to complex chemical transformation. The chosen methods depend entirely on the desired quality of the final product.
Stage 1: Physical Separation (Pre-treatment)
This initial stage removes solids and some water without altering the oil's chemistry.
- Hot-gas Filtration: The most common first step is to filter the hot pyrolysis vapors before they are condensed. This removes fine char and ash particles, which can act as catalysts for unwanted aging reactions in the final liquid.
- Decanting or Centrifugation: After condensation, allowing the oil to settle can separate out an aqueous phase from the organic phase. A centrifuge can accelerate this process, though a complete separation is often difficult.
Stage 2: Chemical Upgrading (Transformation)
This is the most critical stage, where the oil's chemical structure is fundamentally changed to resemble a hydrocarbon.
- Hydrotreating / Hydrodeoxygenation (HDO): This is the most effective and widely studied method. The oil is reacted with hydrogen gas at high temperatures (300-400°C) and pressures over a catalyst. This process cleaves oxygen from the organic molecules, producing water as a byproduct and leaving behind stable hydrocarbons. The result is a high-quality, energy-dense oil that can be a "drop-in" fuel or refinery feedstock.
- Catalytic Cracking: Pyrolysis oil can be introduced into a fluid catalytic cracker (FCC), often co-processed with petroleum gas oil. The catalysts and high temperatures crack the large, oxygenated molecules into smaller, more valuable gasoline-range hydrocarbons. This is an attractive option for existing refineries.
- Esterification: To specifically combat acidity, the oil can be reacted with an alcohol (like ethanol or butanol). This converts the corrosive carboxylic acids into less harmful esters, which also improves the oil's stability.
Understanding the Trade-offs
While chemical upgrading is technically effective, it introduces significant economic and operational hurdles that are critical to consider.
High Cost and Complexity
Processes like HDO are capital-intensive. They require high-pressure reactors, a continuous supply of hydrogen (which is expensive to produce or purchase), and sophisticated catalysts that can deactivate over time. As noted, this complexity and cost are often prohibitive for smaller pyrolysis plants, making it difficult to justify the investment.
Energy Consumption
The upgrading process itself is energy-intensive. The high temperatures and pressures required for HDO and catalytic cracking consume a significant portion of the energy that is ultimately contained in the final fuel, impacting the overall energy balance and efficiency of the system.
Catalyst Challenges
Finding robust catalysts is a major challenge. Pyrolysis oil can quickly foul and deactivate catalysts due to char and heavy polymer deposition. Developing long-lasting, coke-resistant catalysts is a primary focus of ongoing research and adds to the operational cost.
Making the Right Choice for Your Goal
The appropriate purification strategy depends entirely on your end-use application and economic constraints. There is no single "best" method.
- If your primary focus is producing a transportable, refinery-ready fuel: Hydrodeoxygenation (HDO) is the most direct path to creating a stable, high-quality synthetic crude oil, but you must be prepared for its high capital and operational costs.
- If your primary focus is creating valuable chemicals: Fractional distillation can be used to separate the oil into different chemical families (e.g., phenols, anhydrosugars), but this requires a specialized market and a complex separation train.
- If your primary focus is low-cost, on-site energy generation: Minimal upgrading, such as simple filtration to remove char followed by co-firing in a dedicated industrial boiler or furnace, may be the most economical solution.
Ultimately, turning raw pyrolysis oil into a valuable product is a battle against its inherent chemistry, where technical solutions must be constantly weighed against economic reality.
Summary Table:
| Purification Stage | Key Methods | Primary Goal |
|---|---|---|
| Physical Separation | Hot-gas filtration, Centrifugation | Remove solids, char, and some water |
| Chemical Upgrading | Hydrotreating (HDO), Catalytic Cracking, Esterification | Reduce oxygen content, improve stability, and increase energy density |
| Trade-offs | High cost, energy consumption, catalyst challenges | Balance technical effectiveness with economic feasibility |
Ready to optimize your pyrolysis oil purification process? KINTEK specializes in lab equipment and consumables for advanced energy and chemical research. Whether you're exploring hydrotreating catalysts or scaling up filtration systems, our solutions help you achieve higher efficiency and better results. Contact us today to discuss how we can support your laboratory's pyrolysis oil upgrading projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs



















