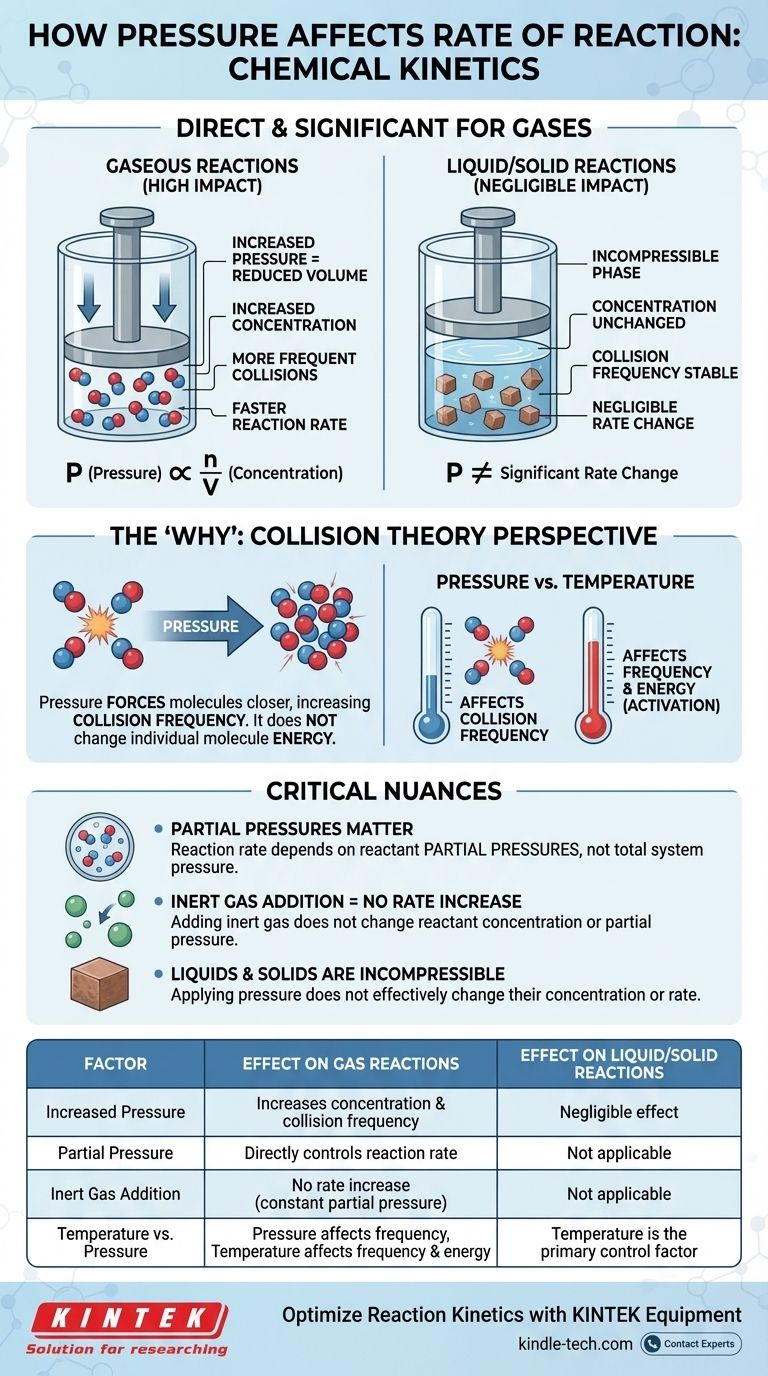
In chemical kinetics, the effect of pressure on reaction rate is direct and significant, but only for reactions involving gases. For a gaseous reaction, increasing the pressure increases the concentration of the reactant molecules. This leads to more frequent collisions between them, which in turn increases the overall rate of the reaction. For reactions occurring purely in liquid or solid phases, pressure has a negligible effect on the rate.
The relationship between pressure and reaction rate is fundamentally a story about concentration. For gases, increasing pressure is simply a physical means of forcing more molecules into the same volume, increasing the frequency of effective collisions and thus accelerating the reaction.

The Fundamental Link: Pressure as a Proxy for Concentration
The core reason pressure affects gaseous reactions lies in its direct relationship with concentration. This principle is best explained by looking at the foundational laws of chemistry.
The Role of the Ideal Gas Law
The Ideal Gas Law, PV = nRT, provides the mathematical link. If we rearrange it to solve for pressure (P = (n/V)RT), we can see that pressure (P) is directly proportional to n/V.
The term n/V represents moles (n) per unit volume (V), which is the very definition of molar concentration. Therefore, as you increase the pressure of a system at a constant temperature, you are directly increasing the concentration of the gas molecules within it.
How Concentration Governs Reaction Rate
A reaction's speed is defined by its rate law, often expressed as rate = k[A]^m[B]^n. In this equation, [A] and [B] represent the concentrations of the reactants.
The rate law shows that the reaction rate is directly dependent on the concentration of its reactants. A higher concentration means a faster rate. By connecting this to the gas law, the chain of events becomes clear: increasing pressure increases concentration, which in turn increases the reaction rate.
The "Why" Behind the Change: A Collision Theory Perspective
Rate laws tell us what happens, but Collision Theory explains why it happens on a molecular level. For a reaction to occur, reactant particles must collide with both sufficient energy and the correct orientation.
The Principle of Collision Frequency
A chemical reaction is the result of countless molecular collisions. The more collisions that happen per second, the more opportunities there are for a successful reaction to occur.
How Pressure Boosts Collisions
Increasing the pressure on a gas reduces the volume it occupies, forcing the molecules closer together. This dramatically increases their collision frequency—the number of times they bump into each other per unit of time.
While pressure does not change the energy of individual molecules (that is the role of temperature), it multiplies the number of total collision events. This increase in frequency leads to a proportional increase in successful, reaction-causing collisions.
Understanding the Nuances and Trade-offs
While the general rule holds, an expert must understand the specific conditions under which it applies and, more importantly, when it does not.
The Critical Role of Partial Pressures
In a mixture of gases, the overall reaction rate depends on the partial pressures of the specific reactants, not the total pressure of the system. The partial pressure is the pressure that a single gas would exert if it alone occupied the entire volume.
This means you can increase the total pressure by adding an inert gas (like argon or nitrogen) to the reaction vessel. However, since this does not change the concentration or partial pressure of the actual reactants, it will not increase the reaction rate.
The Insignificant Effect on Liquids and Solids
Pressure has a negligible impact on reaction rates in condensed phases (liquids and solids). These states of matter are already considered incompressible.
The molecules in liquids and solids are already packed tightly together. Applying external pressure does not significantly decrease the distance between them or change their concentration. Therefore, it is not an effective lever for changing their reaction rates.
Pressure vs. Temperature
It's crucial to distinguish the effects of pressure and temperature.
- Pressure primarily affects the frequency of collisions.
- Temperature affects both the frequency of collisions (molecules move faster) and, more importantly, the energy of each collision.
Raising the temperature increases the fraction of molecules that possess the minimum required activation energy, making it a far more powerful factor for increasing reaction rates than pressure.
Making the Right Choice for Your System
Understanding this principle allows you to control reaction outcomes based on your specific goals. Consider the following when designing or optimizing a chemical process.
- If your primary focus is to maximize the speed of a gaseous reaction: Increasing the system's pressure by reducing its volume is a direct and effective method to boost throughput.
- If your primary focus is to control a reaction in a gas mixture: You must manage the partial pressures of the reactants, as simply adding an inert gas to raise total pressure will not accelerate your target reaction.
- If your primary focus is to alter the rate of a reaction in a liquid or solid: Manipulating pressure is not a viable strategy; you should focus on changing the temperature, concentration of dissolved species, or using a catalyst.
By understanding that pressure is a proxy for concentration in gases, you gain precise control over the kinetics of your system.
Summary Table:
| Factor | Effect on Gas Reactions | Effect on Liquid/Solid Reactions |
|---|---|---|
| Increased Pressure | Increases concentration & collision frequency | Negligible effect (incompressible) |
| Partial Pressure | Directly controls reaction rate | Not applicable |
| Inert Gas Addition | No rate increase (constant partial pressure) | Not applicable |
| Temperature vs. Pressure | Pressure affects frequency; Temperature affects frequency & energy | Temperature is the primary control factor |
Need to precisely control your chemical reactions? KINTEK specializes in high-quality lab equipment, including pressure reactors and gas handling systems, to help you optimize reaction kinetics and achieve superior results in your laboratory. Contact our experts today to discuss your specific application and discover the right solution for your needs.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Electric Split Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
People Also Ask
- What role does an autoclave play in simulating PWR conditions? Advanced Material Validation for Nuclear Safety
- What is the purpose of using a high-temperature hydrothermal reactor? Enhance Iodine@Activated Carbon Cathode Synthesis
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield



















