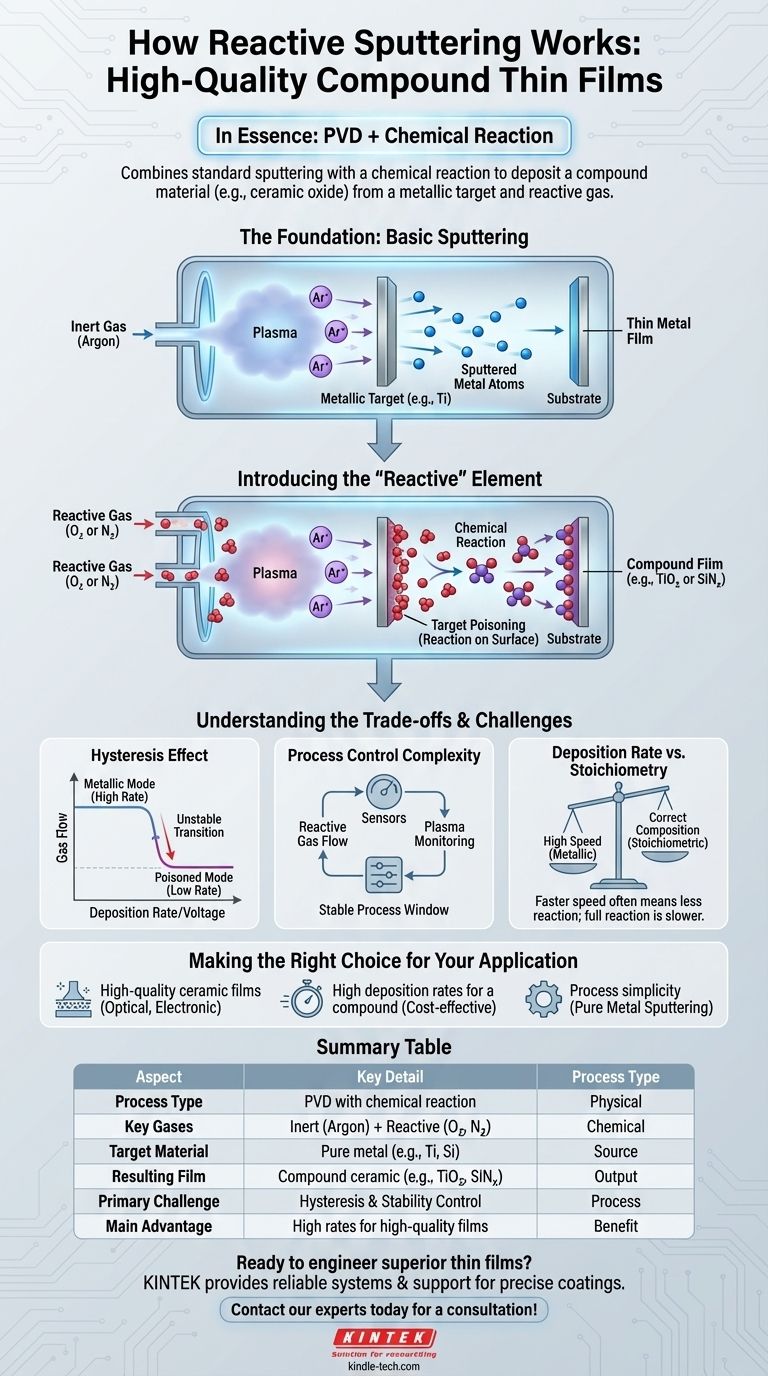
In essence, reactive sputtering is a physical vapor deposition (PVD) technique that combines the mechanics of standard sputtering with a chemical reaction. Instead of depositing a pure material, you sputter a metallic target in a vacuum chamber that also contains a small amount of a reactive gas, like oxygen or nitrogen. This process forces the sputtered metal atoms to chemically bond with the gas, forming a new compound material, such as a ceramic oxide or nitride, directly onto your substrate.
Reactive sputtering is a powerful method for creating high-quality compound thin films, like ceramics, from a simple metallic source. It allows you to leverage the high deposition rates of metal sputtering while producing materials that would otherwise be difficult or slow to deposit directly.

The Foundation: How Basic Sputtering Works
To understand reactive sputtering, we must first establish the principles of standard sputtering. The process occurs within a vacuum chamber and involves three key elements.
The Plasma Environment
First, the chamber is pumped down to a high vacuum and then backfilled with a small amount of an inert gas, most commonly argon. A strong electric field is applied, which ionizes this gas and creates a sustained glow discharge, or plasma.
The Bombardment Process
Within this plasma, positive argon ions are accelerated at high speeds toward a negatively charged plate of source material, known as the target or cathode. These energetic ions act like atomic-scale sandblasters, striking the target with enough force to knock off, or "sputter," individual atoms of the target material.
Deposition on the Substrate
These ejected target atoms travel through the vacuum chamber and condense onto a component, called the substrate, forming a thin, uniform film of the target material.
Introducing the "Reactive" Element
Reactive sputtering builds directly upon this foundation by introducing one critical change: the addition of a second gas.
Adding the Reactive Gas
Alongside the inert argon gas, a controlled amount of a reactive gas—typically **oxygen (O₂) or nitrogen (N₂) **—is introduced into the chamber. The goal is no longer to deposit the pure target metal, but to synthesize a new compound.
Where the Chemical Reaction Occurs
The sputtered metal atoms react with the reactive gas to form a compound film. This chemical reaction can happen in three places: on the surface of the target, in the plasma during transit, or, most commonly, on the surface of the substrate as the film grows.
Forming the Compound Film
The result is a fully-formed compound deposited as a thin film. For example, by sputtering a titanium (Ti) target in the presence of oxygen, you create a titanium dioxide (TiO₂) film. Sputtering a silicon (Si) target with nitrogen gas produces a silicon nitride (SiNₓ) film.
Understanding the Trade-offs
While powerful, reactive sputtering introduces process complexities that require careful management. The interaction between the sputtering rate and the chemical reaction is a delicate balance.
The Hysteresis Effect
The most significant challenge is a phenomenon known as hysteresis. As you increase the flow of reactive gas, the process can abruptly switch from a high-rate "metallic mode" (not enough reaction) to a low-rate "poisoned mode," where the target surface becomes fully coated in the compound, drastically reducing the sputter rate. This can make the process unstable and difficult to control.
Process Control Complexity
Because of hysteresis, maintaining the perfect balance of reactive gas is critical. Too little gas results in a film that isn't fully reacted (e.g., a metallic-looking oxide). Too much gas "poisons" the target, slows deposition to a crawl, and can lead to arcing and process instability. This requires sophisticated feedback control systems for gas flow and plasma monitoring.
Deposition Rate vs. Stoichiometry
There is a direct trade-off between deposition speed and achieving the correct chemical composition (stoichiometry). The fastest deposition occurs just before the target becomes poisoned, but this is also the most unstable process window. Operating in a fully "poisoned" mode is more stable and ensures complete reaction but is significantly slower.
Making the Right Choice for Your Application
Understanding these principles allows you to decide if reactive sputtering is the correct approach for your goal.
- If your primary focus is high-quality ceramic films: Reactive sputtering is an industry-standard method for producing dense, stoichiometric oxides and nitrides for optical, electronic, and protective applications.
- If your primary focus is high deposition rates for a compound: Sputtering a metallic target in reactive mode is often significantly faster and more cost-effective than RF sputtering from a ceramic target of the same compound.
- If your primary focus is process simplicity: Standard DC or RF sputtering of a pure metal or alloy target is less complex, as it avoids the intricate gas and plasma balancing required for reactive deposition.
By mastering the interplay between the physical sputtering and the chemical reaction, you can precisely engineer the properties of your deposited material.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process Type | Physical Vapor Deposition (PVD) with chemical reaction |
| Key Gases | Inert gas (Argon) + Reactive gas (Oxygen, Nitrogen) |
| Target Material | Pure metal (e.g., Titanium, Silicon) |
| Resulting Film | Compound ceramic (e.g., TiO₂, SiNₓ) |
| Primary Challenge | Hysteresis effect and process stability control |
| Main Advantage | High deposition rates for high-quality compound films |
Ready to engineer superior thin films for your lab?
Reactive sputtering is a powerful but complex technique. KINTEK specializes in lab equipment and consumables, providing the reliable sputtering systems and expert support you need to achieve precise, high-quality coatings for your optical, electronic, or protective applications.
Let's discuss your specific requirements and how we can help optimize your deposition process. Contact our experts today for a consultation!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
People Also Ask
- How are PECVD and CVD different? A Guide to Choosing the Right Thin-Film Deposition Process
- How does plasma vapor deposition work? A Low-Temperature Coating Solution for Sensitive Materials
- What is plasma activated chemical vapor deposition? Enable Low-Temperature Thin Film Deposition
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- What is the temperature of PECVD deposition? Achieve High-Quality Films at Low Temperatures



















