To maintain the temperature of a reaction, you must use a system that can efficiently add or remove heat as needed. This is typically achieved by immersing the reaction vessel in a temperature-controlled liquid bath or by using a specialized jacketed vessel where a thermal fluid is circulated around the exterior. The specific method depends on the target temperature and the scale of the reaction.
The core challenge of temperature control is managing heat transfer. Simple manual methods like ice baths are suitable for basic lab cooling, while automated systems with jacketed vessels and Temperature Control Units (TCUs) provide the precision required for sensitive or large-scale processes.

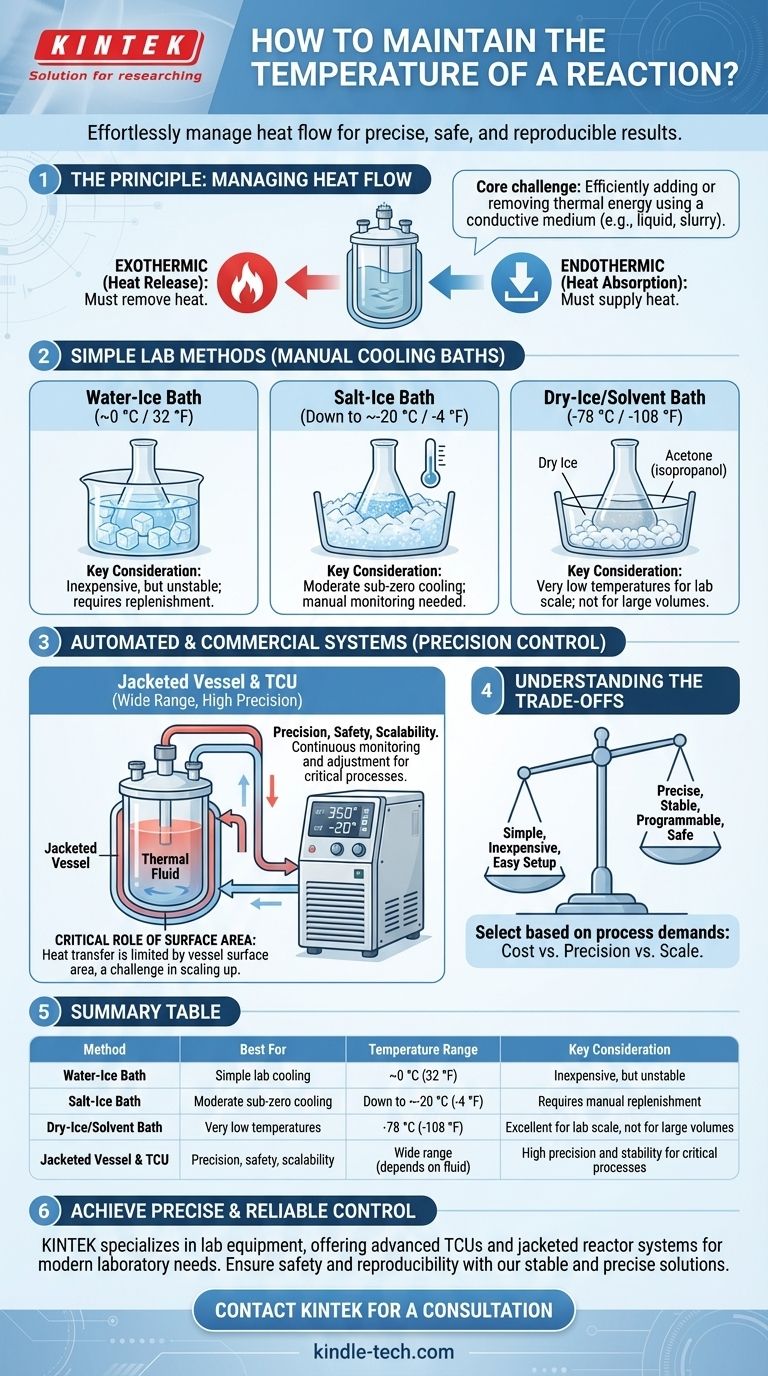
The Principle: Managing Heat Flow
Controlling a reaction's temperature is fundamentally about managing the flow of thermal energy. Reactions can either release heat (exothermic) or absorb it (endothermic), and your control system must counteract these changes.
Why a Thermal Medium is Essential
Air is a poor conductor of heat. To effectively cool or heat a reaction, you must place the vessel in direct contact with a thermal medium—typically a liquid or a slurry—that can transfer heat much more efficiently.
Adding vs. Removing Heat
An exothermic reaction releases heat, which must be constantly removed to prevent the temperature from rising. Conversely, an endothermic reaction absorbs heat from its surroundings, requiring a continuous supply of heat to maintain its temperature.
Common Methods for Temperature Control
The tools for temperature control range from simple lab setups to sophisticated industrial systems.
Simple Cooling Baths (Lab Scale)
For basic cooling, a simple bath is effective. The vessel is placed directly into a container holding a cooling mixture.
- Water-Ice Bath: A mixture of water and ice will naturally hold a temperature of approximately 0 °C (32 °F).
- Salt-Ice Bath: Adding salt to an ice bath disrupts the freezing point of water, allowing for temperatures down to approximately -20 °C (-4 °F).
- Dry-Ice/Solvent Bath: For very low temperatures, solid carbon dioxide (dry ice) is used. Because heat transfer from a solid is inefficient, the dry ice is mixed with a solvent like acetone or isopropanol to create a slurry that maintains -78 °C (-108 °F) and ensures good thermal contact.
Automated and Commercial-Scale Systems
For larger volumes or when high precision is required, manual baths are impractical. These situations call for automated systems.
- The Jacketed Vessel: This is a container with a second outer wall, creating a hollow space or "jacket" around the primary vessel.
- The Temperature Control Unit (TCU): A TCU is a device that heats or cools a thermal fluid (like water, glycol, or specialized oils) and circulates it through the vessel's jacket. It continuously monitors the temperature and makes adjustments to maintain a precise, stable setpoint.
Understanding the Trade-offs
No single method is perfect for every application. The right choice involves balancing cost, precision, and scale.
Manual Baths: Simple but Unstable
Simple ice and dry-ice baths are inexpensive and easy to set up. However, as the reaction proceeds and heat is exchanged, the bath's temperature will change. They require constant monitoring and replenishment (e.g., adding more ice and salt) to maintain a relatively stable temperature.
Jacketed Systems: Precise but Complex
A jacketed vessel paired with a TCU offers unparalleled precision, stability, and programmability. This is critical for safety and reproducibility in commercial production. The trade-off is significant cost and complexity in both equipment and operation.
The Critical Role of Surface Area
In any method, the rate of heat transfer is limited by the surface area of the reaction vessel. A larger vessel has a lower surface-area-to-volume ratio, making it harder to control the temperature of the material in the center. This is a key challenge when scaling up reactions.
Making the Right Choice for Your Reaction
Select your method based on the specific demands of your process.
- If your primary focus is simple, small-scale cooling to 0 °C: A standard water-ice bath is the most practical and economical choice.
- If your primary focus is achieving stable sub-zero temperatures in a lab: A dry-ice/solvent bath is the standard method, but be prepared for manual monitoring.
- If your primary focus is precision, safety, and scalability for a critical process: A jacketed vessel with a Temperature Control Unit is the only reliable solution.
Ultimately, mastering temperature control is fundamental to achieving safe, predictable, and repeatable results in any chemical process.
Summary Table:
| Method | Best For | Temperature Range | Key Consideration |
|---|---|---|---|
| Water-Ice Bath | Simple lab cooling | ~0 °C (32 °F) | Inexpensive, but unstable |
| Salt-Ice Bath | Moderate sub-zero cooling | Down to ~-20 °C (-4 °F) | Requires manual replenishment |
| Dry-Ice/Solvent Bath | Very low temperatures | -78 °C (-108 °F) | Excellent for lab scale, not for large volumes |
| Jacketed Vessel & TCU | Precision, safety, scalability | Wide range (depends on fluid) | High precision and stability for critical processes |
Achieve precise and reliable temperature control for your lab processes.
Scaling up a reaction or working with sensitive materials requires robust temperature management to ensure safety and reproducibility. KINTEK specializes in lab equipment and consumables, offering advanced Temperature Control Units (TCUs) and jacketed reactor systems designed for the exacting needs of modern laboratories.
Our solutions provide the stability and precision you need to master heat transfer in your chemical processes. Contact us today to discuss how we can support your laboratory's specific temperature control challenges.
Contact KINTEK for a consultation
Visual Guide
Related Products
- 100L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 10L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
- 10L Chilling Circulator Cooling Water Bath Low Temperature Constant Temperature Reaction Bath
- 100L Chilling Circulator Cooling Water Circulator for Low Temperature Constant Temperature Reaction Bath Water Bath Cooling
People Also Ask
- For which types of substances are water baths and chillers considered ideal? Essential Care for Sensitive Samples
- How does a high-precision constant temperature circulator contribute to mineral dissolution kinetic studies?
- What role does a high-precision constant temperature circulating water bath play in AEM research? Stability & Control
- What is the function of a constant temperature water bath in CO2 absorption kinetics? Achieve High-Precision Research
- How does a water bath work? Master Precise and Gentle Heating for Your Lab



















