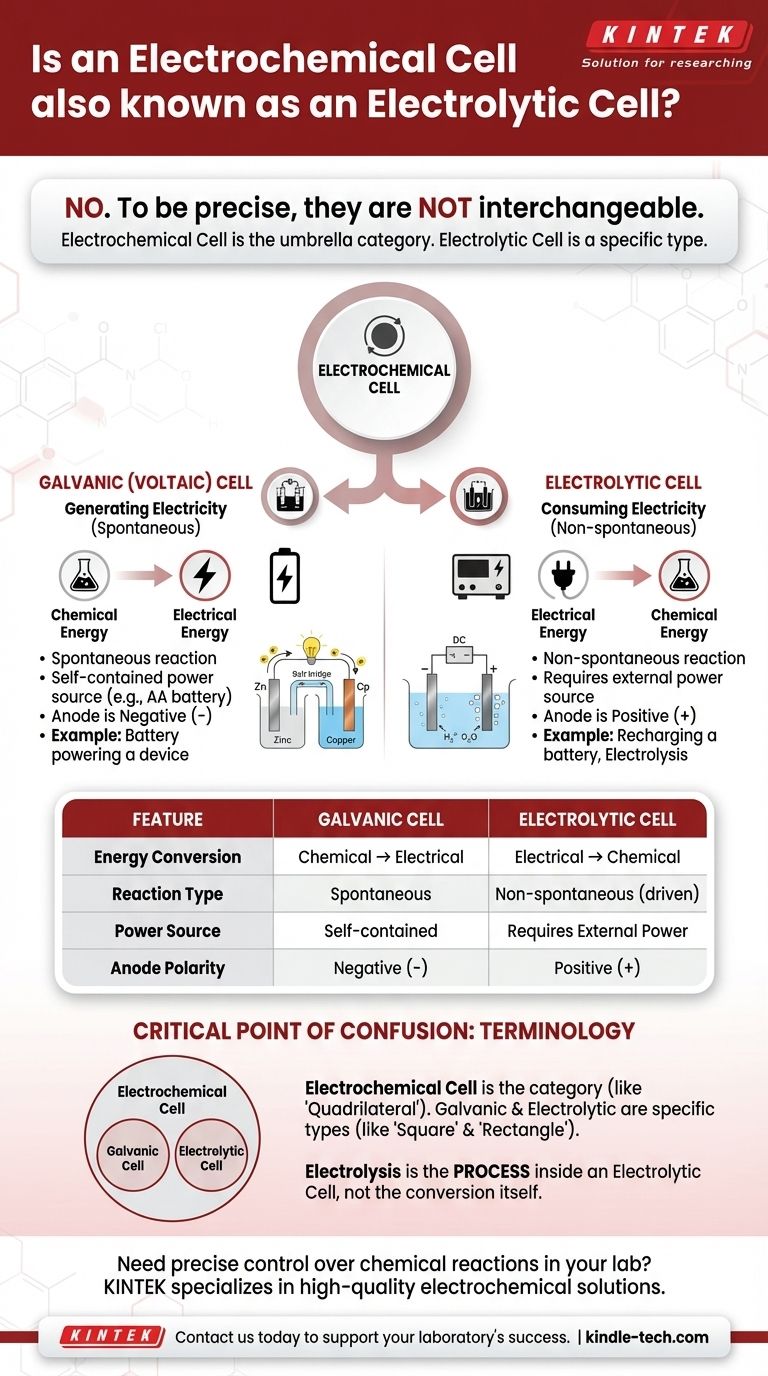
To be precise, no. An electrolytic cell is a specific type of electrochemical cell, but the two terms are not interchangeable. "Electrochemical cell" is the broad, umbrella category for any device that converts chemical energy into electrical energy or vice versa. Electrolytic cells are the type that specifically use electrical energy to cause a chemical reaction.
The core distinction comes down to the direction of energy conversion. A galvanic (or voltaic) cell spontaneously releases energy from a chemical reaction to create electricity. An electrolytic cell consumes electricity from an external source to force a chemical reaction to occur.
The Fundamental Distinction: Energy Flow
The purpose of an electrochemical cell dictates its classification. The primary question to ask is whether the cell is producing energy or consuming it.
Galvanic (Voltaic) Cells: Generating Electricity
A galvanic cell, also known as a voltaic cell, harnesses a spontaneous chemical reaction.
The reactants within the cell have a natural tendency to react, releasing energy in the process. This energy is captured as an electric current.
Think of a standard AA battery in a remote control. It is a galvanic cell, converting its internal chemical energy into the electrical energy needed to power the remote.
Electrolytic Cells: Consuming Electricity
An electrolytic cell uses an external source of electrical energy (like a power supply) to drive a non-spontaneous chemical reaction.
Without this external power, the chemical reaction would not occur on its own. The electricity forces the reaction to proceed against its natural tendency.
A common example is electrolysis, such as splitting water into hydrogen and oxygen gas. Another is recharging a lithium-ion battery; you are using electricity to reverse the discharge reaction and restore its chemical potential.
Key Identifiers for Each Cell Type
Beyond the direction of energy flow, a few technical characteristics help differentiate the two cell types.
The Role of an External Power Source
This is the most straightforward indicator. If a device is self-contained and acting as a power source, it is a galvanic cell.
If the device requires being plugged into an external power source to function, it is an electrolytic cell.
Anode and Cathode Polarity
While the definitions of anode (where oxidation occurs) and cathode (where reduction occurs) remain constant, their electrical charge (polarity) flips between the two cell types.
In a galvanic cell, the anode is the negative (-) terminal and the cathode is the positive (+) terminal.
In an electrolytic cell, the external power source reverses this. The anode becomes the positive (+) terminal and the cathode becomes the negative (-) terminal.
The Critical Point of Confusion: Terminology
The primary source of confusion stems from the overlapping but distinct nature of the terms. Failing to differentiate them leads to fundamental misunderstandings.
"Electrochemical Cell" is the Umbrella Term
Think of "electrochemical cell" as the general category, like "quadrilateral."
Galvanic cells and electrolytic cells are the specific types within that category, much like "square" and "rectangle" are specific types of quadrilaterals. An electrolytic cell is always an electrochemical cell, but not all electrochemical cells are electrolytic.
The Misuse of "Electrolysis"
Electrolysis is the process of using electricity to drive a chemical reaction. This process takes place inside an electrolytic cell.
Some definitions incorrectly state that electrolysis is the conversion of chemical to electrical energy. This is the opposite of the truth. Electrolysis is fundamentally about using electricity to create a chemical change.
Making the Right Distinction for Your Goal
To apply this knowledge correctly, simply identify the primary function of the device you are analyzing.
- If your focus is on a device that is powering something (a remote, a phone, a clock): You are dealing with a galvanic (voltaic) cell, which converts stored chemical energy into electricity.
- If your focus is on a process that uses electricity to create a substance (recharging a battery, plating metal, producing chlorine gas): You are dealing with an electrolytic cell, which uses electrical energy to force a chemical change.
- If you are describing the general field or a device with dual capabilities (like a rechargeable battery): The term "electrochemical cell" is the correct and most inclusive choice.
Understanding the direction of energy conversion is the key to mastering this fundamental concept in chemistry and engineering.
Summary Table:
| Feature | Galvanic (Voltaic) Cell | Electrolytic Cell |
|---|---|---|
| Energy Conversion | Chemical → Electrical | Electrical → Chemical |
| Reaction Type | Spontaneous | Non-spontaneous (driven) |
| Power Source | Self-contained (e.g., battery) | Requires external power supply |
| Anode Polarity | Negative (-) terminal | Positive (+) terminal |
| Common Example | AA battery in a remote | Recharging a lithium-ion battery |
Need precise control over chemical reactions in your lab? Understanding the right type of electrochemical cell is crucial for your research and processes. At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your specific electrochemical needs. Whether you're working with galvanic cells for energy generation or electrolytic cells for synthesis, our solutions ensure accuracy and reliability. Contact us today to discuss how we can support your laboratory's success!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control



















