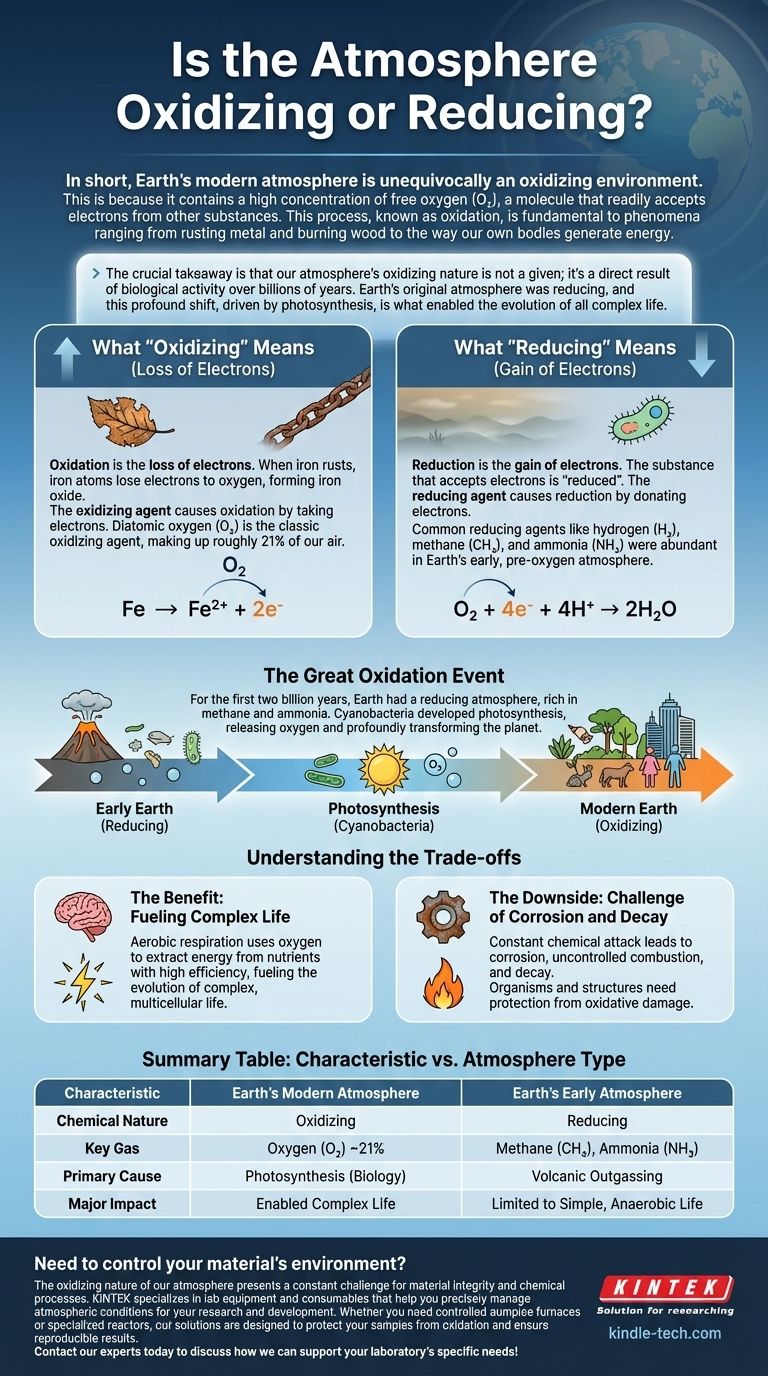
In short, Earth’s modern atmosphere is unequivocally an oxidizing environment. This is because it contains a high concentration of free oxygen (O₂), a molecule that readily accepts electrons from other substances. This process, known as oxidation, is fundamental to phenomena ranging from rusting metal and burning wood to the way our own bodies generate energy.
The crucial takeaway is that our atmosphere's oxidizing nature is not a given; it's a direct result of biological activity over billions of years. Earth's original atmosphere was reducing, and this profound shift, driven by photosynthesis, is what enabled the evolution of all complex life.

What "Oxidizing" and "Reducing" Really Mean
To grasp the state of our atmosphere, we first need to define the core chemical principles at play. These terms describe the transfer of electrons between molecules.
The Role of Electrons
Oxidation is the loss of electrons. When iron rusts, iron atoms lose electrons to oxygen, forming iron oxide.
Reduction is the gain of electrons. The substance that accepts the electrons is said to be "reduced." In the rusting example, oxygen gains electrons from the iron, so the oxygen is reduced.
Identifying an Oxidizing Agent
An oxidizing agent (or oxidant) is a substance that causes oxidation by taking electrons from something else.
Because of its powerful tendency to attract and accept electrons, diatomic oxygen (O₂) is the classic and most significant oxidizing agent in our environment.
Identifying a Reducing Agent
A reducing agent (or reductant) is a substance that causes reduction by donating its electrons to something else.
Common reducing agents include hydrogen (H₂), methane (CH₄), and ammonia (NH₃). These were abundant in Earth's early, pre-oxygen atmosphere.
The Dominant Force: Why Our Atmosphere is Oxidizing
The chemical character of our atmosphere is dictated by one dominant molecule that makes up roughly 21% of the air we breathe.
The Oxygen Factor
The presence of about 21% free oxygen is the primary reason our atmosphere is oxidizing. Oxygen is highly electronegative, meaning its atoms have a strong pull on electrons. This makes O₂ chemically aggressive and ready to react with—and oxidize—a vast range of other elements and compounds.
The Great Oxidation Event
Our planet was not always like this. For the first two billion years of its history, Earth had a reducing atmosphere, virtually free of O₂ and rich in gases like methane and ammonia.
This changed with the evolution of cyanobacteria. These microorganisms developed photosynthesis, a process that uses sunlight to create energy and releases oxygen as a waste product. Over hundreds of millions of years, this biological activity slowly and profoundly transformed the entire planet, pumping enormous quantities of O₂ into the air and shifting it to its modern oxidizing state.
Understanding the Trade-offs
An oxygen-rich, oxidizing environment presents both a powerful advantage and a persistent chemical challenge.
The Benefit: Fueling Complex Life
The primary benefit is aerobic respiration. This metabolic process uses oxygen to extract energy from nutrients with incredible efficiency—far more than the anaerobic (oxygen-free) processes that preceded it. This massive energy surplus is what fueled the evolution of complex, multicellular life, including us.
The Downside: The Challenge of Corrosion and Decay
The "cost" of an oxidizing atmosphere is constant chemical attack. This is the force behind corrosion (like rust), uncontrolled combustion (fire), and the decay of organic matter.
Living organisms must invest energy in sophisticated antioxidant defenses to protect their cells from oxidative damage, while any structure we build must be designed to resist the relentless effects of atmospheric oxygen.
Key Takeaways for Different Contexts
Your approach to this fact will depend on your field and your goals.
- If your primary focus is chemistry or materials science: Recognize that any substance exposed to our air is in a constant battle against oxidation, a critical factor influencing material selection, preservation, and durability.
- If your primary focus is biology or geology: Understand that the shift from a reducing to an oxidizing atmosphere was arguably the single most significant environmental change in Earth's history, dictating the entire subsequent course of evolution.
- If your primary focus is astrobiology: Use the presence of substantial free oxygen in an exoplanet's atmosphere as a leading biosignature, as it is difficult to maintain without a powerful, planet-wide biological source.
Understanding our atmosphere's oxidizing nature is to understand the fundamental chemical engine that drives both life and decay on our world.
Summary Table:
| Characteristic | Earth's Modern Atmosphere | Earth's Early Atmosphere |
|---|---|---|
| Chemical Nature | Oxidizing | Reducing |
| Key Gas | Oxygen (O₂) ~21% | Methane (CH₄), Ammonia (NH₃) |
| Primary Cause | Photosynthesis (Biology) | Volcanic Outgassing |
| Major Impact | Enabled Complex Life | Limited to Simple, Anaerobic Life |
Need to control your material's environment? The oxidizing nature of our atmosphere presents a constant challenge for material integrity and chemical processes. KINTEK specializes in lab equipment and consumables that help you precisely manage atmospheric conditions for your research and development. Whether you need controlled atmosphere furnaces or specialized reactors, our solutions are designed to protect your samples from oxidation and ensure reproducible results. Contact our experts today to discuss how we can support your laboratory's specific needs!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How does a high-temperature atmosphere sintering furnace contribute to UO2 fuel pellets? Achieve High-Density Precision
- Why is it necessary to control atmosphere during sintering? Prevent Oxidation and Control Material Properties
- What is the mechanism of a reduction atmosphere furnace in exsolution? Precision Control for Perovskite Nanoparticles
- What are the benefits of using an inert gas to prevent oxidation? Protect Materials and Boost Quality
- What is used to provide an inert atmosphere for welding? Master the Shield for Perfect Welds
- What is a controlled atmosphere temperature treatment system? A Guide to Precision Heat Treatment
- What is a controlled atmosphere heat treatment furnace? Achieve Superior Metallurgical Results
- What is an inert atmosphere heat treatment? Protect Your Metals from Oxidation & Decarburization



















