On the contrary, tungsten is not brittle at high temperatures. It is exceptionally strong and ductile, which is precisely why it is selected for some of the most demanding high-temperature applications, such as rocket nozzles and furnace heating elements. Its reputation for brittleness comes from its behavior at room temperature.
Tungsten's defining characteristic is its transition from a brittle state at or near room temperature to a highly ductile, workable state at elevated temperatures. Understanding this behavior, known as the ductile-to-brittle transition, is the key to successfully using this powerful material.
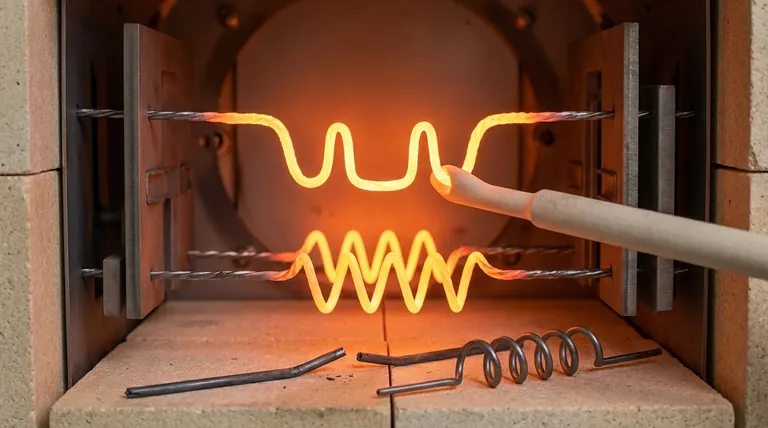
The Core Concept: Tungsten's Temperature-Dependent Behavior
Tungsten's mechanical properties change dramatically with temperature. This duality is its most critical feature from an engineering perspective.
Brittleness at Room Temperature
At ambient temperatures, pure tungsten has a body-centered cubic (BCC) crystal structure that restricts the movement of atoms. This makes it very hard but also brittle, meaning it is prone to fracturing under impact or stress rather than bending.
The Ductile-to-Brittle Transition Temperature (DBTT)
Tungsten has a specific temperature threshold, the DBTT, above which its behavior fundamentally changes. For pure tungsten, this transition typically begins around 400°C (752°F).
Above this temperature, the atoms have enough thermal energy to move more freely within the crystal lattice. The material loses its brittleness and becomes ductile and malleable, allowing it to be bent, stretched, and formed without cracking.
Ductility and Strength at High Temperatures
As the temperature increases well beyond its DBTT, tungsten's "excellent high-temperature strength," as noted in material science data, becomes its dominant feature. It can withstand immense stress at temperatures where most other metals would have already melted.
Why Tungsten Excels in Extreme Heat
Tungsten’s performance at high temperatures is rooted in its fundamental physical properties.
Unmatched Melting Point
With the highest melting point of any pure metal at 3422°C (6192°F), tungsten maintains its solid structure long after steel, titanium, and superalloys have liquefied.
High Creep Resistance
Creep is the tendency of a material to slowly deform over time under constant stress at high temperatures. Tungsten's strong atomic bonds give it exceptional resistance to creep, ensuring structural stability in applications like heating elements that operate for thousands of hours.
Thermal and Chemical Stability
Tungsten performs best in a vacuum or inert atmosphere. The references note its suitability for high vacuum levels because, in the presence of oxygen, it will oxidize rapidly at high temperatures. It also shows excellent resistance to corrosion from molten alkali metals.
Understanding the Practical Trade-offs
While tungsten is a superior high-temperature material, its properties create specific engineering challenges that must be managed.
The Challenge of Cold Brittleness
The primary trade-off is its brittleness at room temperature. This makes machining, forming, or handling tungsten components difficult. Parts must often be heated to be worked, and designs must avoid sharp corners or stress points that could lead to fracture when cold.
The Necessity of a Controlled Atmosphere
Tungsten's utility in air is severely limited at high temperatures. Above approximately 400-500°C, it begins to form a volatile oxide that causes the material to quickly sublimate and fail. This is why applications like incandescent bulb filaments are encased in a vacuum or inert gas.
Alloying to Modify Properties
Pure tungsten's properties are not always ideal. It is often alloyed with other elements, such as rhenium, to lower its DBTT. This makes the resulting alloy more ductile at lower temperatures, improving its machinability and resistance to fracture during handling.
Making the Right Choice for Your Application
Selecting tungsten requires a clear understanding of the entire operational temperature cycle, from fabrication to end-use.
- If your primary focus is structural integrity above 1000°C: Tungsten is an exceptional choice, as its ductility at these temperatures prevents the brittle fracture seen when it is cold.
- If your primary focus is ease of fabrication at room temperature: You must account for tungsten's inherent brittleness, which often requires specialized heating, cutting techniques, or the use of more ductile tungsten alloys.
- If your application involves high temperatures in an oxygen-rich environment: Pure tungsten is unsuitable due to rapid oxidation; you must operate it in a vacuum or inert atmosphere to ensure its longevity.
Ultimately, harnessing tungsten's power lies in designing for its strong, ductile high-temperature state while respecting its brittle nature when cold.
Summary Table:
| Property | Behavior at Room Temperature | Behavior at High Temperatures (Above ~400°C) |
|---|---|---|
| Ductility | Brittle, prone to fracture | Highly ductile and malleable |
| Strength | Very hard but brittle | Exceptional strength, resists creep |
| Key Characteristic | Brittle fracture under stress | Can be bent and formed without cracking |
| Primary Use Limitation | Difficult to machine and handle | Must be used in vacuum/inert atmosphere to prevent oxidation |
Ready to harness the power of tungsten for your high-temperature applications? KINTEK specializes in providing high-performance lab equipment and consumables, including tungsten heating elements and components designed for extreme environments. Our experts can help you select the right materials to ensure durability and efficiency in your laboratory processes. Contact us today to discuss your specific needs and discover how our solutions can elevate your research and production capabilities.
Visual Guide

Related Products
- Thermally Evaporated Tungsten Wire for High Temperature Applications
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Boron Nitride (BN) Ceramic Rod for High Temperature Applications
- Engineering Advanced Fine Alumina Al2O3 Ceramic Rod Insulated for Industrial Applications
- High-Purity Titanium Foil and Sheet for Industrial Applications
People Also Ask
- What are the common materials used as heating elements? Find the Right Material for Your Temperature Needs
- What is the disadvantage of using tungsten? The High Cost and Difficulty of Fabrication
- Do heating elements degrade over time? Understanding the Inevitable Decay for Better Performance
- Why are high-power electric heating rods used in in-situ catalyst reaction cells? Ensure Precision & Thermal Stability
- Why are tungsten-rhenium (W/Re) thermocouples selected for monitoring the combustion synthesis of ferroalloys? - Up to 2400°C
- How do resistive heating elements work? Unlock the Science of Efficient Heat Generation
- What are the causes of failure of heating elements? Prevent Downtime with Proper Operation
- What function do molybdenum disilicide heating elements perform? Precision Heat for Pulverized Coal Research



















