In short, platinum electrodes are used where a combination of chemical inertness, conductivity, and catalytic activity is essential. Their primary applications are in sensitive electrochemical analysis, long-term biomedical implants, industrial catalysis, and as durable components in microelectronics. They are valued for their stability and resistance to corrosion in harsh chemical and biological environments.
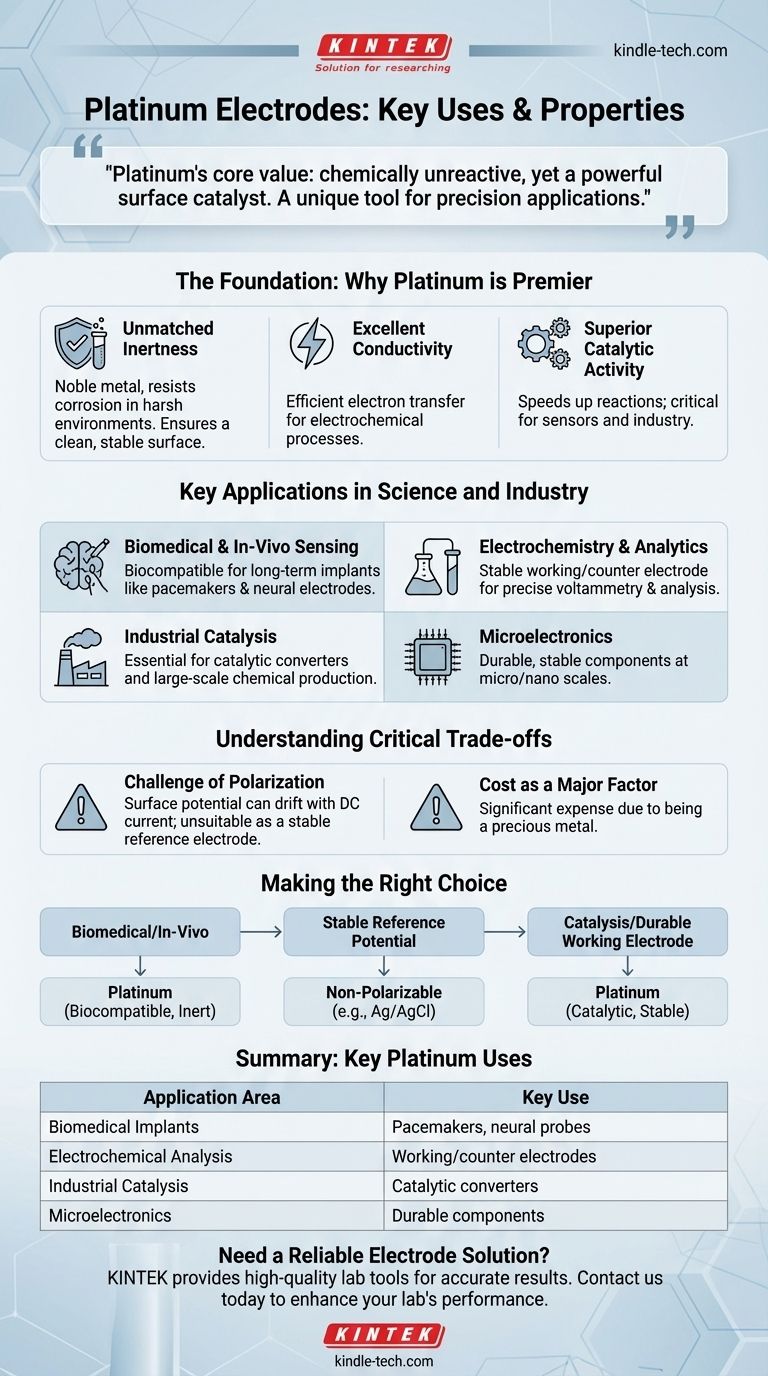
Platinum's core value as an electrode material stems from a powerful paradox: it is almost completely unreactive chemically, yet it is an excellent catalyst for speeding up reactions at its surface. This makes it a uniquely stable and active tool for a range of precision applications.

The Foundation: Why Platinum is a Premier Electrode Material
Platinum possesses a unique set of physical and chemical properties that make it a go-to material for demanding electrode applications. Understanding these fundamentals explains its widespread use.
Unmatched Chemical Inertness
Platinum is a noble metal, meaning it strongly resists oxidation and corrosion, even in aggressive acids or biological systems.
This inertness ensures the electrode itself does not interfere with the chemical reactions being studied or catalyzed. It provides a clean, stable surface.
Excellent Electrical Conductivity
Like most metals, platinum is an excellent conductor of electricity. This allows it to efficiently transfer electrons to or from a solution, which is the fundamental job of any electrode.
Superior Catalytic Activity
Despite being chemically inert, platinum's surface is exceptionally effective at catalyzing (speeding up) a wide variety of chemical reactions.
This is critical in sensors, where it helps the target substance react, and in industrial processes where reaction efficiency is paramount.
Key Applications in Science and Industry
The combination of these core properties makes platinum indispensable in several high-stakes fields.
Biomedical and In-Vivo Sensing
Platinum is highly biocompatible, meaning it does not provoke a significant immune response when placed inside the body.
This makes it the material of choice for long-term implants like pacemaker leads, cochlear implants, and neural electrodes used for deep brain stimulation.
Electrochemistry and Analytics
In a laboratory setting, platinum is often used as a working electrode or counter electrode in techniques like voltammetry.
Its stable and predictable behavior provides a reliable surface for studying the electrochemical properties of different chemical species.
Industrial Catalysis
Platinum's catalytic prowess is harnessed on a massive scale in industry. Most notably, it is a key component in automotive catalytic converters.
It is also used in the production of fertilizers, high-octane gasoline, and other commodity chemicals where it accelerates reactions without being consumed.
Microelectronics
Platinum's stability and conductivity make it suitable for creating reliable, long-lasting contacts and components at the micro and nano scales.
Understanding the Critical Trade-offs
While powerful, platinum is not the perfect choice for every situation. Its properties come with specific limitations that every engineer and scientist must understand.
The Challenge of Polarization
Platinum is a polarizable electrode. This means its surface potential can drift or change when a direct current (DC) is passed through it.
For this reason, it is generally unsuitable as a reference electrode, whose job is to maintain an absolutely stable potential. Materials like Silver/Silver Chloride (Ag/AgCl) are superior for that specific task.
The Condition for DC Measurement
Platinum can be used for measuring DC potentials, but only under zero-current conditions.
This is achievable in systems with very high impedance, like the input of a pH meter, where virtually no current is drawn from the electrode.
Cost as a Major Factor
As a precious metal, platinum is significantly more expensive than other electrode materials. This cost is often the primary barrier to its use in applications where a less expensive alternative could suffice.
Making the Right Choice for Your Application
Selecting an electrode material requires matching its properties to your primary objective.
- If your primary focus is biological implants or in-vivo measurement: Platinum's biocompatibility and inertness make it one of the safest and most reliable choices available.
- If your primary focus is a stable reference potential in a current-carrying circuit: Avoid platinum and choose a non-polarizable electrode like Ag/AgCl for superior accuracy.
- If your primary focus is catalysis or a durable, inert working electrode for analysis: Platinum's unique combination of chemical stability and catalytic activity is its greatest strength.
Ultimately, choosing platinum is a decision to prioritize performance and stability, especially in environments where other materials would quickly fail.
Summary Table:
| Application Area | Key Use of Platinum Electrodes |
|---|---|
| Biomedical Implants | Pacemaker leads, neural probes, and cochlear implants due to biocompatibility. |
| Electrochemical Analysis | Working/counter electrodes in lab instruments for precise measurements. |
| Industrial Catalysis | Catalytic converters and chemical production processes. |
| Microelectronics | Durable, stable components and contacts at micro scales. |
Need a Reliable Electrode Solution for Your Lab?
Platinum electrodes are critical for precision and stability in demanding applications. KINTEK specializes in high-quality lab equipment and consumables, providing the reliable tools your laboratory needs to achieve accurate results in electrochemistry, materials science, and biomedical research.
Let our experts help you select the right equipment for your specific requirements.
Contact KINTEK today to discuss your project and discover how our solutions can enhance your lab's performance.
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Electrochemical Sheet Electrode Gold Electrode
People Also Ask
- What are the functions of platinum sheet and Ag/AgCl electrodes in corrosion testing? Master Electrochemical Precision
- What are the standard specifications for platinum wire and rod electrodes? Select the Right Form Factor for Your Experiment
- What is a common use for a platinum sheet electrode? As a Reliable Counter Electrode in Electrochemical Cells
- How can a worn or scratched platinum disk electrode surface be restored? Achieve a Mirror Finish for Reliable Data
- How should a platinum wire/rod electrode be installed? Ensure Accurate Electrochemical Measurements



















