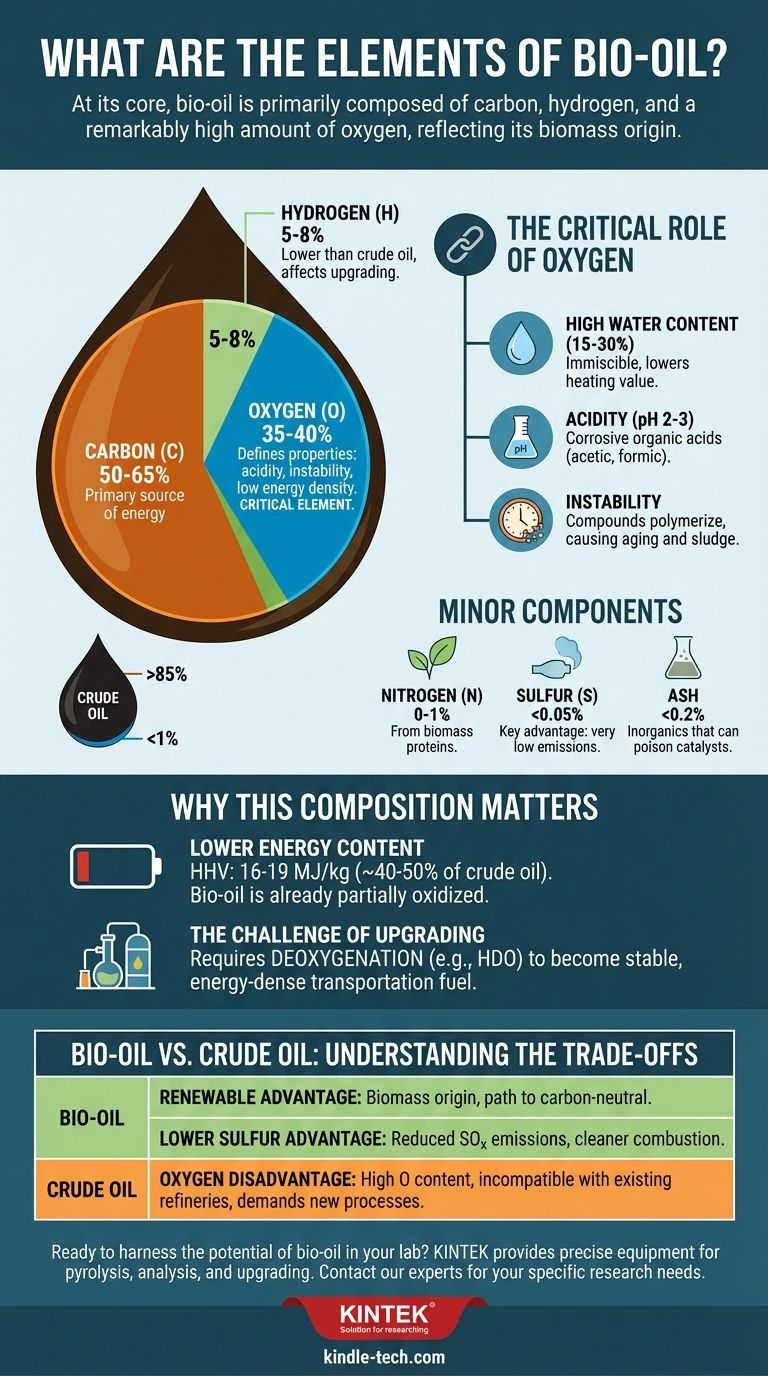
At its core, bio-oil is primarily composed of carbon, hydrogen, and a remarkably high amount of oxygen. Unlike conventional crude oil, which is almost entirely carbon and hydrogen, the elemental makeup of bio-oil is a direct reflection of its biomass origin. This high oxygen content is the single most important factor defining its properties, benefits, and challenges.
The fundamental takeaway is that bio-oil's high oxygen content (typically 35-40% by weight) makes it fundamentally different from fossil fuels. This single elemental characteristic is responsible for its lower energy density, acidity, and instability, dictating the need for specialized processing before it can be used as a drop-in fuel.

A Detailed Look at Bio-Oil's Composition
Bio-oil, also known as pyrolysis oil, is not a single compound but a complex mixture of hundreds of organic molecules. Its elemental composition provides the essential blueprint for understanding its behavior.
The Primary Elemental Makeup
The bulk of bio-oil consists of three main elements:
- Carbon (C): Typically ranges from 50-65%.
- Hydrogen (H): Typically ranges from 5-8%.
- Oxygen (O): Typically ranges from 35-40%.
This composition is starkly different from crude oil, which is often >85% carbon, 10-14% hydrogen, and <1% oxygen.
The Critical Role of Oxygen
The high concentration of oxygen is not just a number; it is the source of bio-oil's most distinct properties. The oxygen is bound within various chemical functional groups, creating compounds like acids, aldehydes, ketones, and phenols.
This leads directly to several key characteristics:
- High Water Content: Bio-oil can contain 15-30% water, which is immiscible with hydrocarbons.
- Acidity: The presence of organic acids (like acetic and formic acid) gives bio-oil a low pH (typically 2-3), making it corrosive.
- Instability: Reactive compounds like aldehydes and ketones can polymerize over time, causing the bio-oil to thicken, age, and form sludge.
Nitrogen, Sulfur, and Ash
Beyond the primary elements, bio-oil contains smaller but significant components.
- Nitrogen (N): Typically present at 0-1%, originating from proteins and other compounds in the biomass.
- Sulfur (S): Usually very low, often below 0.05%, which is a significant advantage over many high-sulfur fossil fuels.
- Ash: This is the inorganic portion of the original biomass, containing elements like potassium, sodium, and calcium. While a small fraction (<0.2%), these metals can poison catalysts used in upgrading processes.
Why This Composition Matters
Understanding the elemental and chemical makeup of bio-oil is critical because it directly dictates its practical applications and limitations.
Impact on Energy Content
The energy in a fuel is released by breaking carbon-hydrogen and carbon-carbon bonds and forming new bonds with oxygen. Because bio-oil contains so much oxygen already, it is partially oxidized.
This means its Higher Heating Value (HHV) is significantly lower—around 16-19 MJ/kg. This is roughly 40-50% of the energy value of conventional fuel oil (42-44 MJ/kg).
The Challenge of Upgrading
To be used as a transportation fuel, bio-oil must be "upgraded." The primary goal of upgrading is deoxygenation—removing oxygen to make the oil more stable, less corrosive, and energy-dense.
This is most commonly done through processes like hydrodeoxygenation (HDO), which uses hydrogen and a catalyst to strip oxygen atoms from the molecules, producing water as a byproduct.
Understanding the Trade-offs: Bio-Oil vs. Crude Oil
Viewing bio-oil as a direct substitute for crude oil is a common mistake. It is a distinct chemical feedstock with its own set of advantages and disadvantages driven by its elemental composition.
The Oxygen Disadvantage
The high oxygen content is the primary technical barrier. It makes bio-oil incompatible with existing refinery infrastructure built for hydrocarbons, demanding new processes and materials to handle its corrosivity and instability.
The Renewable Advantage
Bio-oil's defining benefit is its origin. It is derived from renewable biomass, such as wood, agricultural residues, or algae. As part of the biogenic carbon cycle, it offers a path toward carbon-neutral liquid fuels.
The Lower Sulfur Advantage
The inherently low sulfur content of most biomass means bio-oil is also low in sulfur. This significantly reduces sulfur oxide (SOx) emissions upon combustion, a major contributor to acid rain and air pollution.
Making the Right Choice for Your Goal
How you interpret bio-oil's elemental composition depends entirely on your objective.
- If your primary focus is direct combustion for heat: The lower heating value and high water content must be accounted for, and equipment must be made of materials resistant to its acidity.
- If your primary focus is producing transportation fuel: The high oxygen content dictates that a robust and efficient deoxygenation process is the most critical and costly step.
- If your primary focus is producing specialty chemicals: The oxygenated compounds, such as phenols and levoglucosan, are not a problem but are actually valuable products that can be extracted.
Understanding that bio-oil is an oxygenated, aqueous-phase chemical mixture—not a hydrocarbon—is the first principle for successfully harnessing its potential.
Summary Table:
| Element | Typical Composition (wt%) | Key Impact |
|---|---|---|
| Oxygen (O) | 35-40% | Defines properties: acidity, instability, low energy density |
| Carbon (C) | 50-65% | Primary source of energy |
| Hydrogen (H) | 5-8% | Lower than crude oil, affects upgrading |
| Water (H₂O) | 15-30% | Immiscible, lowers heating value |
| Nitrogen (N) | 0-1% | From biomass proteins |
| Sulfur (S) | <0.05% | Key advantage: very low emissions |
| Ash | <0.2% | Inorganics that can poison catalysts |
Ready to harness the potential of bio-oil in your lab?
Understanding the complex chemistry of bio-oil is the first step. KINTEK specializes in providing the precise lab equipment and consumables you need for pyrolysis, analysis, and upgrading processes. Whether you're researching fuel production, chemical extraction, or material characterization, our reliable tools help you achieve accurate and reproducible results.
Let us equip your laboratory for success. Contact our experts today to discuss your specific bio-oil research needs and discover the right solutions from KINTEK.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Bottle Oil Fume Sampling Tube
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Copper Foam
- Iridium Dioxide IrO2 for Water Electrolysis
People Also Ask
- What are the disadvantages of oil sludge? Avoid Catastrophic Engine Damage and Costly Repairs
- What is the lifespan of a filter media? Understand the 3 Types for Optimal Filtration
- What is the problem with oil sludge? It's the precursor to catastrophic engine failure.
- What is the difference between PPF and coating? Armor vs. Slick Shell for Your Car
- Is PTFE corrosion resistant? Discover the Ultimate Chemical Resistance for Your Lab




