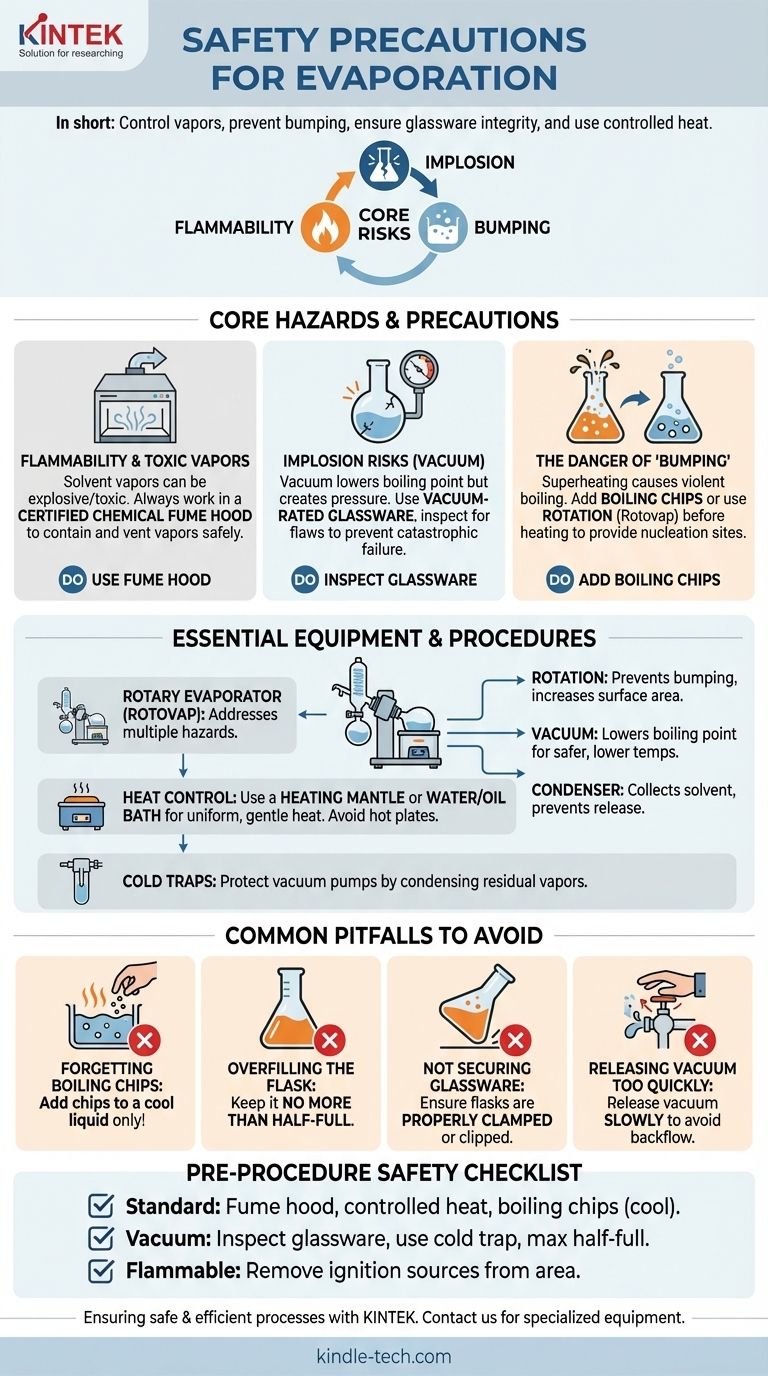
In short, safe evaporation requires controlling flammable vapors with a fume hood, preventing violent boiling (bumping) with boiling chips or rotation, ensuring glassware can withstand pressure changes, and using a controlled heat source. These steps are critical because the process intentionally turns volatile, and often hazardous, liquids into a gaseous state.
The core principle of evaporation safety is managing the three primary risks: the flammability of solvent vapors, the potential for glassware implosion under vacuum, and the sudden release of pressure from superheating.

Analyzing the Core Hazards
To work safely, you must first understand the specific dangers inherent in the evaporation process. Each piece of safety equipment and every step in the procedure is designed to mitigate one of these fundamental risks.
Flammability and Toxic Vapors
Evaporation's entire purpose is to create vapor. Many common organic solvents (like ethanol, acetone, or hexane) are highly flammable, and their vapors can form explosive mixtures with air.
This is why all evaporations must be performed inside a certified chemical fume hood. The hood contains these vapors and vents them safely away, preventing them from accumulating in the lab and finding an ignition source.
Implosion Risks with Vacuum Evaporation
Applying a vacuum significantly lowers a solvent's boiling point, making evaporation faster and more efficient. However, it also puts immense atmospheric pressure on the outside of the glassware.
A tiny, unseen flaw or crack can cause the glass to fail catastrophically, resulting in a dangerous implosion that sends glass shards flying. Always use glassware specifically rated for vacuum, such as heavy-walled filter flasks or round-bottom flasks, and inspect it carefully for any damage before use.
The Danger of "Bumping"
When a liquid is heated without a point for bubbles to form, it can become superheated past its boiling point. The subsequent, instantaneous boiling is called bumping—a violent burst of vapor that can splash hot, hazardous liquid out of the flask.
To prevent this, you must provide nucleation sites. This is achieved by adding boiling chips or a magnetic stir bar to the liquid before heating, or by using a rotary evaporator (rotovap), where the flask's rotation constantly agitates the liquid.
Essential Equipment and Procedures
Proper technique and equipment are your primary tools for controlling the hazards. Following these procedures transforms a potentially dangerous task into a routine and safe one.
The Fume Hood: Your Primary Defense
The fume hood is non-negotiable. It contains flammable and toxic vapors and provides a physical barrier between you and the apparatus. Ensure the sash is lowered to the appropriate level to maintain proper airflow and protection.
Rotary Evaporators (Rotovaps)
A rotovap is a specialized tool that makes evaporation safer and more efficient by addressing multiple hazards at once.
- Rotation: The constant spinning of the flask creates a large surface area for evaporation and provides continuous agitation, which prevents bumping.
- Vacuum: A connected pump lowers the pressure, allowing for evaporation at a lower, safer temperature.
- Condenser: Chilled coils cause the solvent vapor to condense back into a liquid, which is collected in a separate flask. This prevents vapors from escaping into the lab or damaging the vacuum pump.
Heat Control
Never heat a flask directly on a hot plate. This creates a localized hot spot that can lead to bumping or thermal stress on the glass. Instead, use a heating mantle or a water/oil bath, which provides gentle, uniform heat to the entire flask.
Cold Traps
When using a vacuum pump, a cold trap (often filled with dry ice and a solvent) should be placed between your apparatus and the pump. This trap condenses any residual solvent vapors before they can enter and damage the expensive pump mechanism or contaminate its oil.
Common Pitfalls to Avoid
Simple mistakes are often the cause of laboratory accidents. Being aware of these common errors is crucial for maintaining safety.
Forgetting Boiling Chips or Stirring
This is the most common cause of bumping. Crucially, you must add boiling chips to a cool liquid. Adding them to a hot liquid can trigger the violent boiling you were trying to prevent.
Overfilling the Flask
A flask used for evaporation should never be more than half-full. This provides headspace and prevents the solvent from splashing or bumping up into the condenser, which would contaminate your final product.
Not Securing Your Glassware
Ensure all flasks are properly clamped. A round-bottom flask in a heating bath can become buoyant and tip over. On a rotovap, use a keck clip to secure the flask to the apparatus to prevent it from detaching and falling into the bath.
Releasing the Vacuum Too Quickly
When the evaporation is complete, release the vacuum slowly. A sudden influx of air can cause the collected solvent in the receiving flask to be violently pushed back into your primary flask, ruining your sample.
A Pre-Procedure Safety Checklist
Before you begin, run through this mental checklist to ensure you have accounted for the primary risks.
- If your primary focus is any standard evaporation: Always work in a fume hood, use a controlled heat source, and add boiling chips or a stir bar before you begin heating.
- If your primary focus is vacuum evaporation: Meticulously inspect all glassware for cracks, use a cold trap to protect the pump, and ensure your flask is no more than half-full.
- If your primary focus is handling flammable solvents: Double-check that all potential ignition sources (sparking motors, hot plates) are removed from the fume hood and surrounding area.
By understanding the principles behind the precautions, you can perform any evaporation with confidence and safety.
Summary Table:
| Safety Risk | Key Precaution | Purpose |
|---|---|---|
| Flammable/Toxic Vapors | Use a certified fume hood | Contain and vent hazardous vapors |
| Bumping (Violent Boiling) | Add boiling chips or use a rotary evaporator | Provide nucleation sites for gentle boiling |
| Glassware Implosion | Use vacuum-rated glassware and inspect for flaws | Prevent catastrophic failure under vacuum |
| Heat Control | Use heating mantles or water/oil baths | Apply uniform heat to avoid hot spots |
| Vacuum Pump Protection | Install a cold trap | Condense solvent vapors to protect the pump |
Ensure your lab's evaporation processes are safe and efficient with KINTEK's specialized equipment. We provide reliable rotary evaporators, vacuum-rated glassware, and safety accessories designed to mitigate risks like bumping, implosion, and vapor exposure. Our products help laboratories handling flammable solvents or sensitive samples maintain compliance and protect personnel. Contact us today to discuss your specific evaporation needs and let our experts recommend the right solution for your workflow.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- Custom Machined and Molded PTFE Teflon Parts Manufacturer for Laboratory ITO FTO Conductive Glass Cleaning Flower Basket
- Custom PTFE Teflon Parts Manufacturer for Centrifuge Tube Racks
- High-Purity Titanium Foil and Sheet for Industrial Applications
People Also Ask
- What inspection should be performed on a PTFE cleaning basket before use? A 3-Step Protocol for Safe, Effective Cleaning
- What are the common specifications and shapes for PTFE cleaning baskets? Maximize Chemical Purity & Process Integrity
- What precautions should be taken regarding the physical handling and loading of a PTFE cleaning basket? Prevent Damage and Ensure Process Integrity
- What is the maximum operating temperature for a PTFE cleaning basket? Avoid Catastrophic Failure at 260°C
- What is the correct way to place items into a PTFE cleaning basket? Master the Art of Perfect, Repeatable Cleaning



















