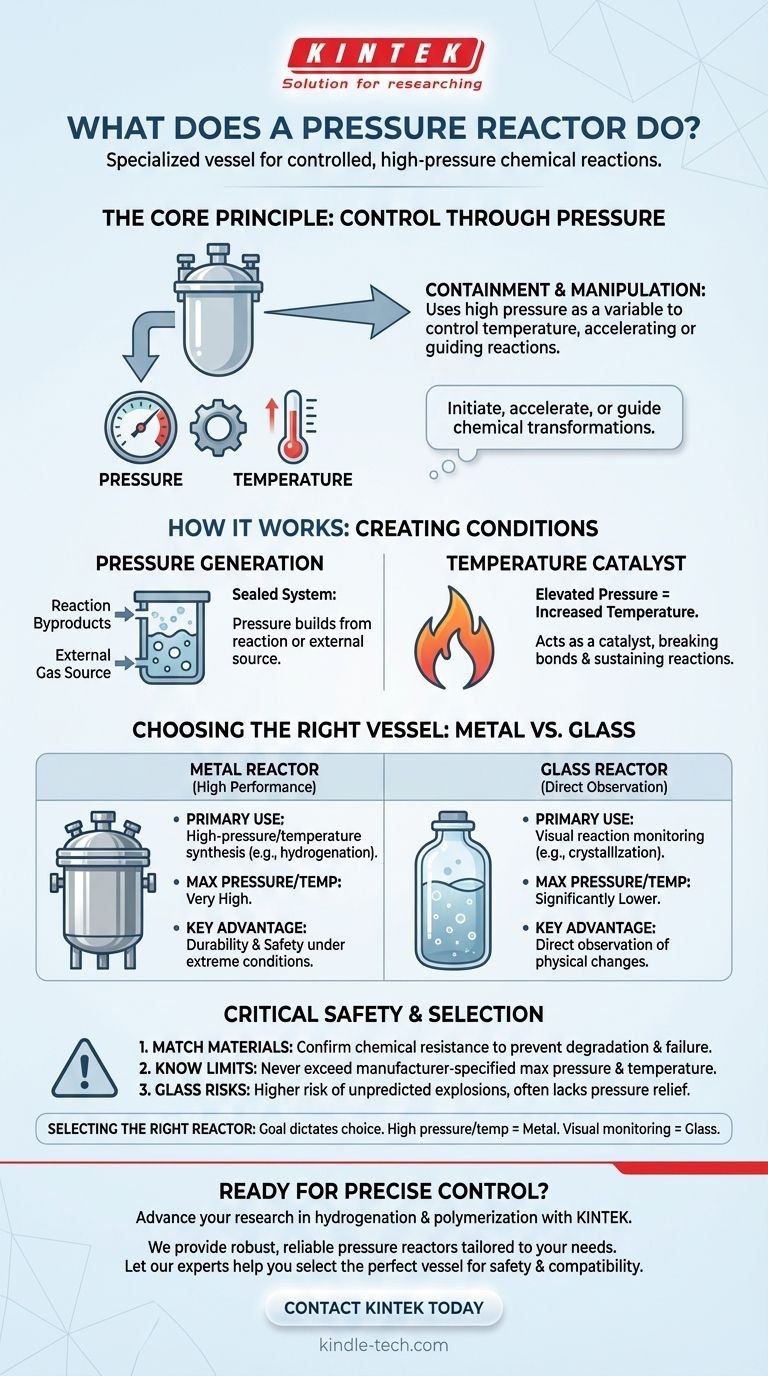
At its core, a pressure reactor is a specialized vessel designed to safely contain chemical reactions that occur at pressures higher than the surrounding atmosphere. This controlled, high-pressure environment is a powerful tool for chemists and engineers to initiate, accelerate, or guide chemical transformations that would be difficult or impossible under normal conditions.
The fundamental purpose of a pressure reactor is not just to contain pressure, but to use that pressure as a variable to manipulate the reaction's temperature and environment, thereby controlling the outcome of a chemical process.

The Core Principle: Using Pressure to Control Reactions
A pressure reactor works by creating a sealed system. As the reaction proceeds or as external gases are introduced, the internal pressure builds, directly influencing the physical and chemical conditions inside the vessel.
How Pressure Creates the Necessary Conditions
The pressure within the reactor can be generated by the reaction itself as gaseous byproducts are formed. Alternatively, it can be created by an external source, such as pumping in a specific gas like hydrogen for a hydrogenation reaction.
This elevated pressure serves as a critical control parameter for a variety of chemical processes, including polymerizations, synthesis, and catalytic reactions.
The Role of Increased Temperature
A direct consequence of increasing the pressure inside a sealed vessel is an increase in temperature. This relationship is fundamental to how these reactors function.
The high temperature acts as a catalyst, providing the energy needed to initiate and sustain chemical reactions until they are complete. It helps break down molecular bonds and ensures processes run efficiently.
Choosing the Right Vessel: Metal vs. Glass
The material of the reactor dictates its capabilities and limitations. The choice between a metal or glass vessel is one of the most important decisions an operator will make.
Metal Reactors for High-Performance Needs
Reactors made from materials like stainless steel are the standard for high-pressure and high-temperature applications. They offer superior durability and safety under extreme conditions.
However, it is crucial to verify the chemical compatibility of the specific grade of steel with the reactants to prevent corrosion or unwanted side reactions.
Glass Reactors for Direct Observation
When an operator needs to visually monitor a reaction, a glass pressure reactor is the ideal choice. They are often used for processes like crystallization or polymerizations where observing physical changes is important.
The trade-off for this visibility is a significantly lower pressure rating compared to metal reactors.
Critical Safety Considerations and Trade-offs
Operating any equipment under high pressure demands a rigorous approach to safety. Understanding the limitations of your reactor is not optional; it is essential for preventing accidents.
Matching Materials to Your Chemistry
You must always confirm that the reactor's material is chemically resistant to the substances being used. An incompatible material can degrade, leading to equipment failure and a dangerous release of pressure and chemicals.
Understanding Pressure and Temperature Limits
Every pressure reactor has a maximum operating temperature and pressure rating. Exceeding these manufacturer-specified limits can lead to catastrophic failure. Always verify that your intended reaction conditions are well within the safe operating range of the vessel.
The Inherent Risks of Glass Vessels
While useful for observation, glass reactors carry a higher risk. They can be susceptible to explosions from excessive internal pressure, which can be difficult to predict. Unlike many metal systems, they often lack pressure relief mechanisms.
How to Select the Right Reactor
Your primary process goal should dictate your choice of equipment.
- If your primary focus is high-pressure or high-temperature synthesis: Choose a metal reactor, ensuring its material grade is compatible with your specific chemical process.
- If your primary focus is observing the reaction progress: A glass pressure reactor is the correct tool, but you must operate it cautiously within its lower, well-defined pressure limits.
Ultimately, a pressure reactor provides you with the precise environmental control required to achieve your desired chemical outcome.
Summary Table:
| Feature | Metal Reactor | Glass Reactor |
|---|---|---|
| Primary Use | High-pressure/temperature synthesis | Visual reaction monitoring |
| Max Pressure/Temp | Very High | Lower |
| Key Advantage | Durability & Safety | Direct Observation |
| Key Consideration | Chemical Compatibility | Pressure Limit Safety |
Ready to achieve precise control over your chemical processes?
A pressure reactor is essential for advancing research in hydrogenation, polymerization, and high-temperature synthesis. At KINTEK, we specialize in providing robust and reliable lab equipment, including a full range of pressure reactors tailored to your specific chemical and safety requirements.
Our experts will help you select the perfect vessel—whether durable metal for extreme conditions or transparent glass for direct observation—ensuring compatibility and safety for your unique application.
Enhance your lab's capabilities and accelerate your R&D. Contact KINTEK today to find the ideal pressure reactor for your needs!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- What role does a high-temperature and high-pressure reactor play in CoFe2O4/Fe synthesis? Unlock Core-Shell Precision
- Why is a high-pressure hydrothermal autoclave preferred for the synthesis of high-crystallinity nanocatalysts?
- What is the significance of using a high-pressure reactor when evaluating the stability of metal oxide catalysts? Find Out Now
- What is the function of a stainless steel fixed-bed reactor in the coconut shell pyrolysis process? Enhance Lab Yields
- What is the advantage of using high-pressure hydrothermal reactors to treat biomass waste? Efficient Resource Recovery
- What is the function of high-temperature and high-pressure reactors in SCWO? Explore Material Science Insights
- Why use precision-machined stainless steel liners in parallel reactors? Optimize Catalyst Evaluation Consistency
- Why are corrosion-resistant chemical reactors essential for the hydrometallurgical leaching of platinum?



















