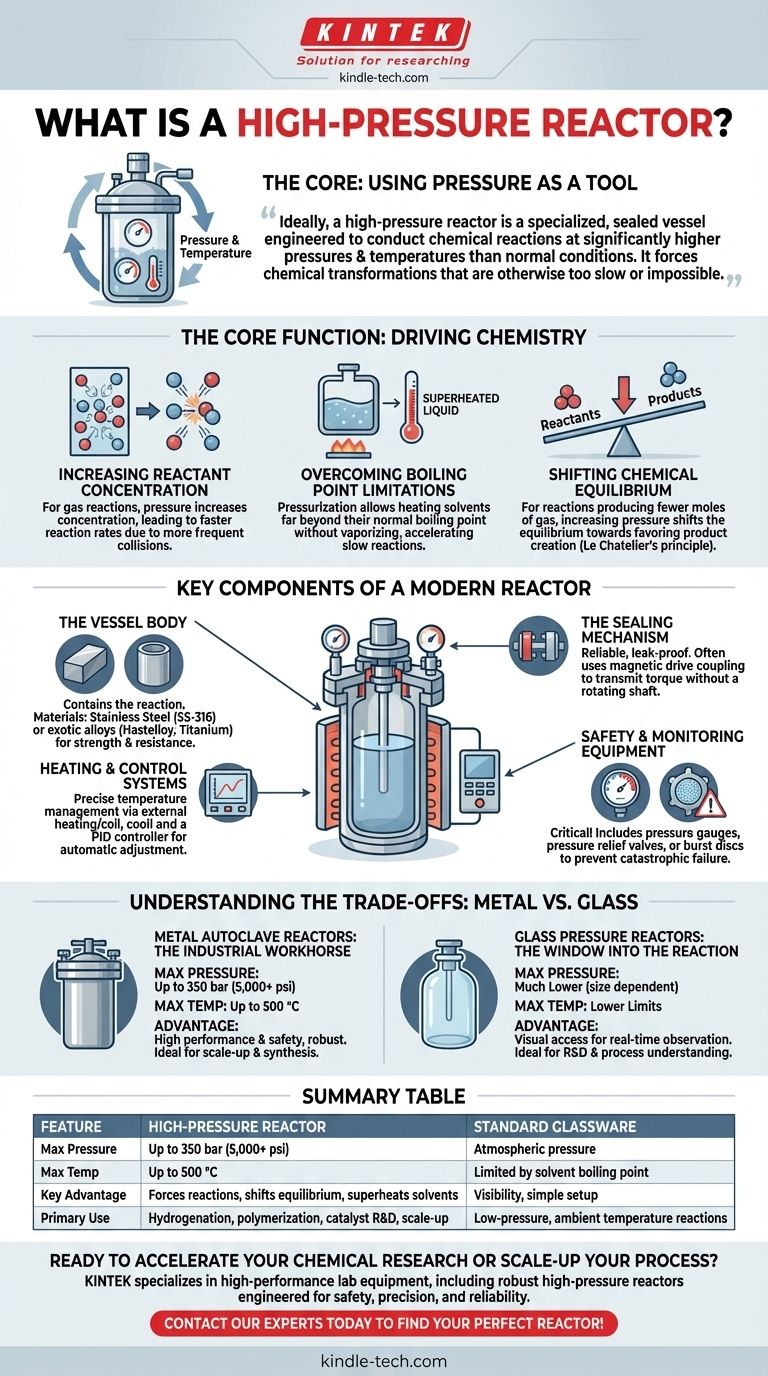
In essence, a high-pressure reactor is a specialized, sealed vessel engineered to safely conduct chemical reactions at pressures and temperatures significantly higher than normal atmospheric conditions. This controlled environment is crucial for forcing chemical transformations that would otherwise be too slow, inefficient, or impossible to achieve in standard laboratory glassware.
The core purpose of a high-pressure reactor is not just to contain pressure, but to use it as a powerful tool. By manipulating pressure and temperature, chemists and engineers can overcome activation energy barriers, increase reaction rates, and shift chemical equilibria to favor desired products.

The Core Function: Using Pressure to Drive Chemistry
A high-pressure reactor creates an artificial environment where the fundamental rules of a chemical reaction can be manipulated. This is primarily achieved by controlling pressure and temperature.
Increasing Reactant Concentration
For reactions involving gases, like hydrogenation or carbonylation, pressure is the most direct way to increase a reactant's concentration. According to basic chemical principles, higher concentrations lead to more frequent molecular collisions and, therefore, a faster reaction rate.
Overcoming Boiling Point Limitations
Many reactions require high temperatures to proceed at a practical speed. By pressurizing the vessel, a solvent can be heated far beyond its normal boiling point without vaporizing. This "superheated" liquid phase is an excellent medium for accelerating slow reactions.
Shifting Chemical Equilibrium
Le Chatelier's principle states that a system at equilibrium will adjust to counteract any change. For reactions that produce fewer moles of gas than they consume, increasing the system pressure will shift the equilibrium to favor the creation of more product.
Key Components of a Modern Reactor
While designs vary, most high-pressure reactors share a common set of critical components that ensure safety and precise control.
The Vessel Body
This is the main chamber that contains the reaction. It is constructed from materials chosen for their strength and resistance to chemical attack at high temperatures and pressures. Common materials include stainless steel (SS-316) for general use and more exotic alloys like Hastelloy, Inconel, or Titanium for highly corrosive environments.
The Sealing Mechanism
A reliable, leak-proof seal is paramount. Modern reactors often use a magnetic drive coupling. This design transmits torque from an external motor to an internal stirrer through a magnetic field, eliminating the need for a rotating shaft to pass through the vessel head. This removes a common failure point and ensures a perfect seal.
Heating and Control Systems
Precise temperature is managed by an external heating mantle or an internal coil. A PID controller (Proportional-Integral-Derivative) is the standard for this task, as it can automatically and accurately maintain the desired reaction temperature by adjusting the power to the heater.
Safety and Monitoring Equipment
This is the most critical system. It includes pressure gauges for monitoring, and more importantly, safety devices like a pressure relief valve or a burst disc. These devices are designed to automatically and safely vent the vessel if the internal pressure exceeds a predetermined safety limit, preventing a catastrophic failure.
Understanding the Trade-offs: Metal vs. Glass
The choice of reactor material is a fundamental decision based on the specific goals of the experiment.
Metal Autoclave Reactors: The Industrial Workhorse
Metal reactors are the default choice for performance and safety. They can withstand extreme conditions, with some models rated for pressures up to 350 bar (over 5,000 psi) and temperatures up to 500 °C. Their robustness makes them suitable for a vast range of applications, from lab-scale synthesis to pilot-plant production.
Glass Pressure Reactors: The Window into the Reaction
The primary advantage of a glass reactor is visual access. Being able to observe color changes, mixing efficiency, or crystallization in real-time is invaluable for process development and research. However, this visibility comes at a significant cost to performance.
Glass reactors have much lower pressure ratings, which are inversely proportional to the vessel's diameter—a larger vessel can withstand less pressure. More critically, they can be susceptible to failure from hard-to-predict pressure spikes, especially if they lack dedicated pressure relief mechanisms.
Making the Right Choice for Your Goal
Selecting the appropriate reactor is a matter of aligning the equipment's capabilities with your primary objective.
- If your primary focus is high performance or scale-up: A metal autoclave made of stainless steel or a suitable alloy is the only safe and effective choice for high pressures, high temperatures, and larger volumes.
- If your primary focus is process understanding and visual observation: A glass pressure reactor is ideal for R&D, but you must operate strictly within its lower pressure and temperature limits and ensure proper safety protocols are in place.
- If your primary focus is general-purpose synthesis: A standard SS-316 metal reactor provides the best balance of chemical resistance, cost, and operational range for common applications like hydrogenation, polymerization, or catalyst screening.
Ultimately, a high-pressure reactor is a precision instrument that transforms pressure from a potential hazard into a controllable variable for chemical innovation.
Summary Table:
| Feature | High-Pressure Reactor | Standard Glassware |
|---|---|---|
| Max Pressure | Up to 350 bar (5,000+ psi) | Atmospheric pressure |
| Max Temperature | Up to 500 °C | Limited by solvent boiling point |
| Key Advantage | Forces reactions, shifts equilibrium, superheats solvents | Visibility, simple setup |
| Primary Use | Hydrogenation, polymerization, catalyst R&D, scale-up | Low-pressure, ambient temperature reactions |
Ready to accelerate your chemical research or scale-up your process? KINTEK specializes in high-performance lab equipment, including robust high-pressure reactors tailored for demanding synthesis, hydrogenation, and catalyst testing. Our reactors are engineered for safety, precision, and reliability, helping you achieve superior results. Contact our experts today to find the perfect reactor for your laboratory's specific needs!
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- What is the advantage of using high-pressure hydrothermal reactors to treat biomass waste? Efficient Resource Recovery
- Why are high-precision pressure sensors and temperature control systems critical for hydrothermal reaction equilibrium?
- What role does a PTFE-lined stainless steel autoclave play in the synthesis of BiOBr precursor nanosheets?
- What are the technical characteristics of PTFE (Teflon) lined hydrothermal reactors? Comparing α-ZrP Synthesis Methods
- What role does a high-pressure reactor play in the hydrodeoxygenation (HDO) of bio-oil? Drive Deep Fuel Upgrading



















