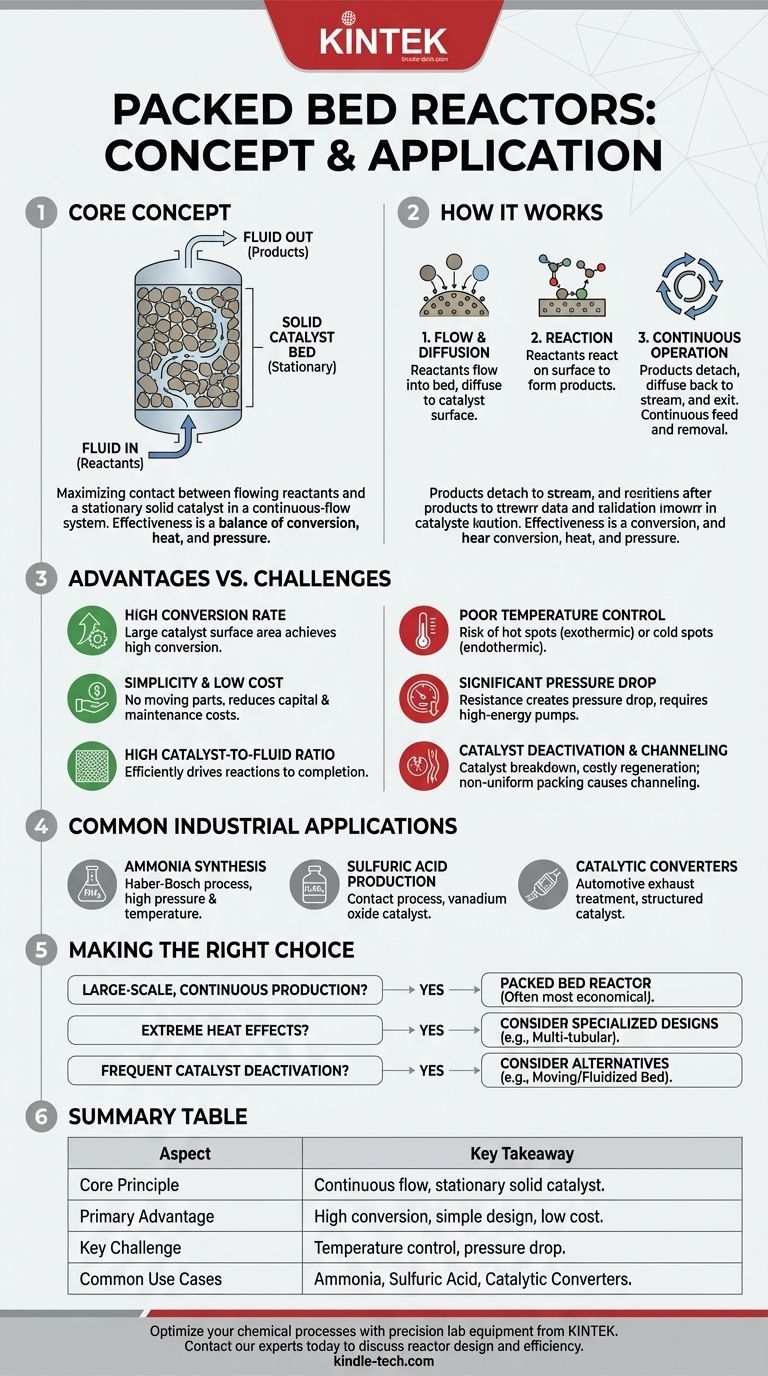
In chemical engineering, a packed bed reactor is a foundational tool for industrial-scale chemical reactions. It is essentially a vessel, typically a cylindrical tube, filled with solid catalyst particles. A fluid, either a gas or liquid, containing the reactants flows through this stationary bed of catalyst, and the chemical reaction occurs on the surface of these particles.
The core concept of a packed bed reactor is to maximize the contact between a flowing reactant stream and a stationary solid catalyst in a simple, continuous-flow system. Its effectiveness is a balance between achieving high chemical conversion, managing heat transfer, and minimizing the pressure drop across the bed.

How a Packed Bed Reactor Works
A packed bed reactor's operation is defined by the interaction between the fluid and the solid catalyst. Understanding this interaction is key to understanding its design and application.
The Core Components
The reactor consists of three primary elements: the vessel itself, the catalyst packing (the "bed"), and the fluid stream. The solid catalyst particles can be shaped as pellets, spheres, or irregular granules and are either dumped randomly into the vessel or, in some advanced applications, arranged in a structured pattern.
The Reaction Mechanism
The process involves several steps. First, reactants in the fluid stream flow into the bed. They must then diffuse from the main fluid stream to the surface of the catalyst particles. Once on the surface, they react to form products, which then detach from the catalyst and diffuse back into the fluid stream to be carried out of the reactor.
Continuous Flow Operation
Unlike a batch reactor where ingredients are mixed and left to react for a set time, a packed bed operates continuously. Reactants are constantly fed into the inlet, and a stream of products is constantly removed from the outlet. The key is to ensure the fluid is distributed evenly across the bed to use all the catalyst effectively.
Key Advantages of the Design
Packed bed reactors are widely used because of several significant advantages over other reactor types, particularly for large-scale production.
High Conversion Rate
By packing a large amount of catalyst surface area into a given volume, these reactors achieve a high rate of conversion. More catalyst means more opportunities for reactants to interact and transform into products as they pass through.
Simplicity and Low Operating Cost
The design is mechanically simple, with no moving parts like the stirrers found in tank reactors. This simplicity reduces both the initial capital investment and ongoing maintenance costs.
High Catalyst-to-Fluid Ratio
The design naturally creates a high ratio of catalyst to the fluid passing through at any given moment. This is highly efficient for driving reactions to completion.
Understanding the Trade-offs and Challenges
Despite its advantages, the packed bed reactor design presents critical challenges that engineers must solve.
Poor Temperature Control
This is often the single biggest challenge. For exothermic reactions (those that release heat), dangerous "hot spots" can form in the bed, potentially damaging the catalyst or causing undesirable side reactions. For endothermic reactions (those that absorb heat), "cold spots" can slow the reaction to a halt.
Significant Pressure Drop
Forcing a fluid through a tightly packed bed of particles creates resistance. This results in a pressure drop from the reactor's inlet to its outlet, requiring more powerful and energy-intensive pumps or compressors to maintain flow.
Catalyst Deactivation
Over time, the catalyst can lose its effectiveness due to poisoning or physical breakdown. Replacing the catalyst, a process called regeneration or repacking, often requires a complete shutdown of the reactor, leading to costly downtime.
The Risk of Channeling
If the catalyst bed is not packed uniformly, the fluid will follow the path of least resistance. This phenomenon, known as channeling, causes large portions of the catalyst bed to be bypassed, drastically reducing the reactor's efficiency.
Common Industrial Applications
The packed bed reactor is a workhorse of the chemical industry, responsible for producing some of the world's most essential chemicals.
Synthesis of Ammonia
The Haber-Bosch process, which produces ammonia for fertilizers, uses packed bed reactors operating at high pressures and temperatures.
Production of Sulfuric Acid
The Contact process uses a packed bed of vanadium oxide catalyst to convert sulfur dioxide into sulfur trioxide, a key step in making sulfuric acid.
Automotive Catalytic Converters
A familiar example is the catalytic converter in your car. Exhaust gases flow through a honeycomb structure coated with precious metal catalysts (a type of structured packed bed) to convert harmful pollutants into less harmful substances.
Making the Right Choice for Your Process
Choosing a reactor type depends entirely on the specifics of the chemical reaction and production goals.
- If your primary focus is large-scale, continuous production with a solid catalyst: A packed bed reactor is often the most economical and straightforward choice.
- If your reaction has extreme heat effects (highly exothermic or endothermic): You must consider specialized designs like multi-tubular reactors or face significant challenges with temperature control.
- If your catalyst deactivates quickly or requires frequent handling: A moving bed or fluidized bed reactor, which allows for easier catalyst removal, might be a more practical alternative.
Understanding these core principles and trade-offs allows you to select and design the most effective reactor for your specific chemical transformation.
Summary Table:
| Aspect | Key Takeaway |
|---|---|
| Core Principle | Continuous flow of reactants through a stationary bed of solid catalyst particles. |
| Primary Advantage | High conversion rates due to a large catalyst surface area; simple design with low operating costs. |
| Key Challenge | Difficult temperature control (risk of hot/cold spots) and significant pressure drop across the bed. |
| Common Use Cases | Ammonia synthesis (Haber-Bosch), sulfuric acid production (Contact process), automotive catalytic converters. |
Optimize your chemical processes with precision lab equipment from KINTEK.
Whether you're scaling up a catalytic reaction or researching new synthesis methods, having the right tools is critical. KINTEK specializes in high-quality lab reactors, furnaces, and consumables tailored to the needs of research and industrial laboratories.
Contact our experts today to discuss how our solutions can enhance your reactor design, improve temperature control, and boost your production efficiency.
Visual Guide
Related Products
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- Why is the use of a corrosion-resistant reactor essential for the acid pretreatment of lignocellulosic materials?
- What is the critical role of an autoclave in the solvothermal synthesis of MOFs? Unlock High-Crystallinity Structures
- Why is a high-pressure solid-phase reaction process necessary for Ag2SnO3? Unlock Unique Modulated Structures
- Why is a high-pressure hydrothermal reactor critical for carbon xerogels? Unlock Superior Electrode Performance
- What specific reaction environment does a high-pressure reactor provide for HTL? Master Biomass to Biocrude Conversion
- Why is a corrosion-resistant stirred reactor necessary for acid leaching? Enhance Rare Earth Element Recovery
- Why are high-pressure reactors or autoclaves necessary for the production of anhydrous magnesite?
- What role does high-pressure hydrogen gas play in formic acid production? Expert Insights into Hydrothermal Synthesis



















