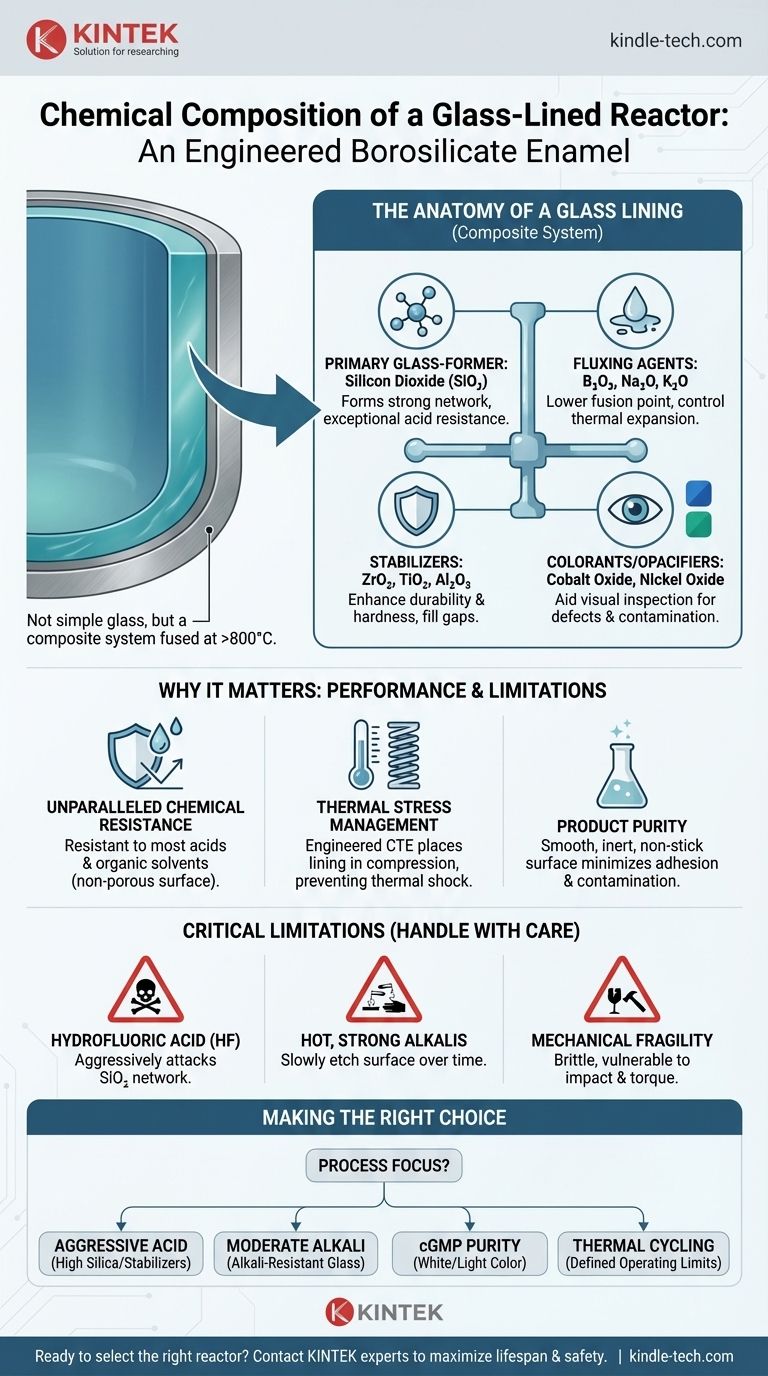
At its core, a glass-lined reactor is not coated with simple glass, but with a highly engineered borosilicate enamel. This material is a complex, multi-component system where the primary chemical is Silicon Dioxide (SiO₂), similar to quartz sand. This base is then blended with various other oxides to achieve extreme chemical resistance and thermal stability far beyond that of any standard glass.
The specific chemical formula of a reactor's glass lining is a closely guarded trade secret that varies by manufacturer and intended use. However, its performance hinges on a strategic blend of glass-forming, fluxing, and stabilizing oxides fused onto a steel substrate in multiple layers. Understanding the function of these components is more critical than knowing their exact percentages.
The Anatomy of a Glass Lining
A glass lining is not a monolithic layer but a composite system. It is created by fusing powdered glass, known as "frit," onto a specially prepared steel surface at extremely high temperatures (over 800°C or 1500°F), creating an inseparable bond.
The Primary Glass-Former: Silicon Dioxide
The backbone of the entire structure is Silicon Dioxide (SiO₂). This compound forms a strong, three-dimensional network of silicon-oxygen bonds, giving the glass its fundamental structure and its exceptional resistance to most acids.
The Fluxing Agents: Lowering the Fusion Point
To fuse the glass to steel at manageable temperatures, fluxing agents are required. These oxides interrupt the pure SiO₂ network, lowering its melting point. Common fluxes include Boron Trioxide (B₂O₃), Sodium Oxide (Na₂O), and Potassium Oxide (K₂O). Boron trioxide also plays a vital role in controlling thermal expansion.
The Stabilizers: Enhancing Durability
Stabilizing oxides are added to improve chemical durability, hardness, and overall mechanical strength. They "fill in" gaps in the glass network, making it less susceptible to chemical attack. Key stabilizers include Zirconium Dioxide (ZrO₂), Titanium Dioxide (TiO₂), and Aluminum Oxide (Al₂O₃).
Colorants & Opacifiers: More Than Aesthetics
Most glass linings are a distinct dark blue, green, or white. This is achieved by adding small amounts of metallic oxides like Cobalt Oxide (for blue) or Nickel Oxide (for green/brown). These colors make it easier to spot surface defects, contamination, or cracks during visual inspection, serving a critical safety and quality function.
Why This Specific Composition Matters
The careful balance of these oxides directly determines the reactor's performance in a harsh chemical processing environment.
Unparalleled Chemical Resistance
The high percentage of SiO₂ and stabilizing oxides creates a highly inert, non-porous surface. It is exceptionally resistant to corrosion from virtually all acids (except hydrofluoric acid) and organic solvents, preventing both damage to the reactor and contamination of the product.
Managing Thermal Stresses
Steel and glass expand and contract at different rates. The glass composition is meticulously formulated so its Coefficient of Thermal Expansion (CTE) is lower than the steel's. This ensures that upon cooling after firing, the steel shell contracts more than the glass, placing the lining in a state of high compression. This compressive stress makes the glass much stronger and more resistant to thermal shock.
Ensuring Product Purity
The extremely smooth, non-stick surface of the glass lining minimizes product adhesion and simplifies cleaning between batches. Its inert nature ensures there is no catalytic effect or leaching of metal ions into the product, which is critical for pharmaceuticals, fine chemicals, and food-grade applications.
Understanding the Trade-offs and Limitations
While remarkably robust, the glass lining's composition also defines its weaknesses. Objectively understanding these is key to ensuring vessel longevity.
The Achilles' Heel: Hydrofluoric Acid
Hydrofluoric acid (HF) and related fluoride compounds are the only acids that aggressively attack the glass lining. The fluoride ion reacts directly with the silicon-oxygen backbone (SiO₂), rapidly dissolving the glass and leading to catastrophic failure.
The Challenge of Strong, Hot Alkalis
While resistant to dilute or cold alkaline solutions, hot and concentrated alkalis (like sodium hydroxide) can slowly etch the glass surface over time. This alkaline corrosion is a known limitation, and specialized alkali-resistant glass formulations exist to mitigate this for specific process conditions.
Mechanical Fragility
Despite its chemical hardness, the lining is still a form of glass. It is brittle and can be easily damaged by mechanical impact (e.g., dropping a tool), excessive torque on connections, or abrasive particles in the process medium.
Making the Right Choice for Your Process
Understanding the chemical composition allows you to ask manufacturers the right questions and select a reactor that aligns with your specific operational needs.
- If your primary focus is aggressive acid service: You need a standard, high-quality formulation rich in silica and stabilizers, as this offers the best protection.
- If your primary focus is moderate alkaline conditions: You must specify an alkali-resistant glass formulation and consult the manufacturer's corrosion charts for your exact temperature and concentration.
- If your primary focus is cGMP and product purity: Prioritize a white or light-colored glass for superior visibility during cleaning and inspection, ensuring no cross-contamination.
- If your primary focus is thermal cycling: Ensure the manufacturer provides clear operating limits for temperature changes to avoid stress cracks, a factor directly tied to the CTE-balancing components in the glass.
Ultimately, viewing the glass lining as an engineered material, not just a coating, is the key to maximizing its lifespan and ensuring the safety and purity of your process.

Summary Table:
| Key Oxide Component | Primary Function |
|---|---|
| Silicon Dioxide (SiO₂) | Forms the glass network; provides exceptional acid resistance. |
| Boron Trioxide (B₂O₃) | Acts as a flux; lowers melting point and controls thermal expansion. |
| Zirconium Dioxide (ZrO₂) | Stabilizer; enhances chemical durability and mechanical strength. |
| Cobalt/Nickel Oxide | Colorant/Opacifier; aids in visual inspection for defects and contamination. |
Ready to select the right glass-lined reactor for your specific chemical process? At KINTEK, we specialize in high-performance lab equipment, including reactors with advanced glass linings tailored for acid resistance, alkali conditions, or cGMP purity. Our experts can help you maximize vessel lifespan and ensure process safety. Contact our team today to discuss your laboratory needs and find the perfect solution!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Super Sealed Electrolytic Electrochemical Cell
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?
- What is the contribution of a hydrothermal reactor to graded pore construction? Precision Templates for TAS
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data



















