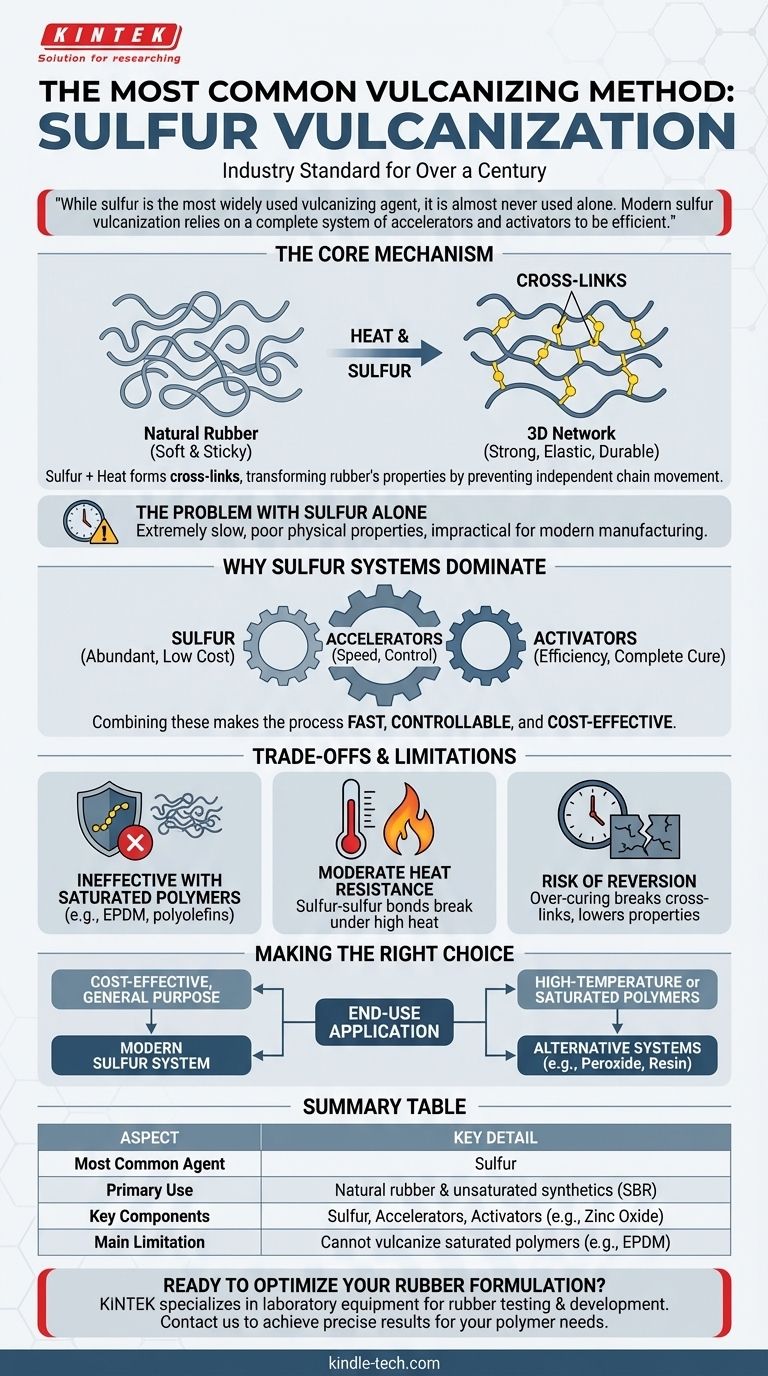
The most common method of vulcanizing is sulfur vulcanization. This foundational process has been the industry standard for over a century, transforming soft, sticky natural rubber into the strong, elastic, and durable material essential for countless applications.
While sulfur is the most widely used vulcanizing agent, it is almost never used alone. Modern sulfur vulcanization relies on a complete system of accelerators and activators to be efficient, highlighting a critical distinction between the core chemical and its practical industrial application.

The Core Mechanism of Sulfur Vulcanization
Vulcanization is the chemical process of converting natural rubber and certain synthetic rubbers into more durable materials by forming cross-links between individual polymer chains.
Creating a Molecular Network
At its core, sulfur vulcanization involves heating rubber in the presence of sulfur. The heat causes the sulfur atoms to form chemical bridges, or cross-links, between the long, tangled polymer chains of the rubber.
This process transforms the material's properties. The newly formed three-dimensional network prevents the polymer chains from moving independently, making the rubber much stronger, more elastic, and resistant to heat and solvents.
The Problem with Sulfur Alone
Using sulfur by itself is a technically possible but commercially unviable process. The reaction is extremely slow, often requiring many hours at high temperatures.
This inefficiency leads to poor physical properties and makes it impractical for modern manufacturing. To overcome this, the industry developed sophisticated cure systems.
Why Sulfur Systems Dominate the Industry
The combination of sulfur with other chemicals, known as accelerators and activators, makes the process fast, controllable, and cost-effective.
The Role of Accelerators
Accelerators are organic compounds that dramatically increase the speed of the vulcanization reaction. They allow the process to be completed in minutes rather than hours, at lower and more controlled temperatures.
The Importance of Activators
Activators, such as zinc oxide and stearic acid, work in tandem with accelerators. They make the accelerators more effective, ensuring an efficient and complete cure that optimizes the final properties of the rubber.
Unmatched Cost-Effectiveness
Sulfur is an abundant and inexpensive raw material. Even when combined with the necessary accelerators and activators, the overall cost of a sulfur vulcanization system is typically lower than alternative methods.
Understanding the Trade-offs and Limitations
Despite its prevalence, sulfur vulcanization is not a universal solution. Its effectiveness and the properties it imparts have inherent limitations.
Ineffectiveness with Saturated Polymers
The primary limitation, as noted in the source material, is that sulfur cannot vulcanize polymers with saturated backbones, such as EPDM or other synthetic polyolefins. These materials lack the necessary chemical sites for sulfur to create cross-links.
Moderate Heat Resistance
Sulfur-cured rubbers generally have a lower maximum service temperature compared to those cured with other systems, like peroxides. The sulfur-sulfur bonds in the cross-links can begin to break down under high heat.
Risk of Reversion
Reversion is a phenomenon that can occur during over-curing, where the cross-links begin to break. This can lead to a significant drop in the rubber's physical properties, such as hardness and tensile strength. Careful control of cure time and temperature is essential to avoid this.
Making the Right Choice for Your Goal
Selecting a vulcanization system requires understanding the end-use application and performance requirements of the final product.
- If your primary focus is cost-effective, general-purpose applications with natural rubber or SBR: The modern sulfur vulcanization system is the undisputed industry standard.
- If your primary focus is high-temperature performance or curing saturated polymers like EPDM: You must explore alternative systems, such as peroxide or resin vulcanization.
Ultimately, understanding that sulfur is the most common agent is only the starting point; recognizing it as part of a complex and adaptable system is key to mastering rubber technology.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Most Common Agent | Sulfur |
| Primary Use | Natural rubber & unsaturated synthetics (e.g., SBR) |
| Key Components | Sulfur, Accelerators, Activators (e.g., Zinc Oxide) |
| Main Limitation | Cannot vulcanize saturated polymers (e.g., EPDM) |
Ready to optimize your rubber formulation and curing process? KINTEK specializes in providing high-quality laboratory equipment and consumables essential for rubber testing and development, from mixers to cure analyzers. Our expertise ensures you achieve precise, reliable results for your specific polymer needs. Contact our experts today to discuss how we can support your laboratory's vulcanization research and quality control!
Visual Guide
Related Products
- Rubber Vulcanizer Vulcanizing Machine Plate Vulcanizing Press for Lab
- Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press 25T 30T 50T
- Manual Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Heated Hydraulic Press Machine with Integrated Manual Heated Plates for Lab Use
- Double Plate Heating Press Mold for Lab
People Also Ask
- What is the necessity of using a laboratory hydraulic pellet press for preparing solid catalysts? Maximize Catalyst Performance
- What are the ingredients used in rubber compounding? A Guide to the Essential Formula
- What is a vulcanizing press? Essential Tool for Rubber Manufacturing and Tire Production
- What is the purpose of a vulcanizing machine? Transform Rubber into High-Performance Parts
- What does vulcanizing a tire do? Achieve a Permanent, Structural Tire Repair



















