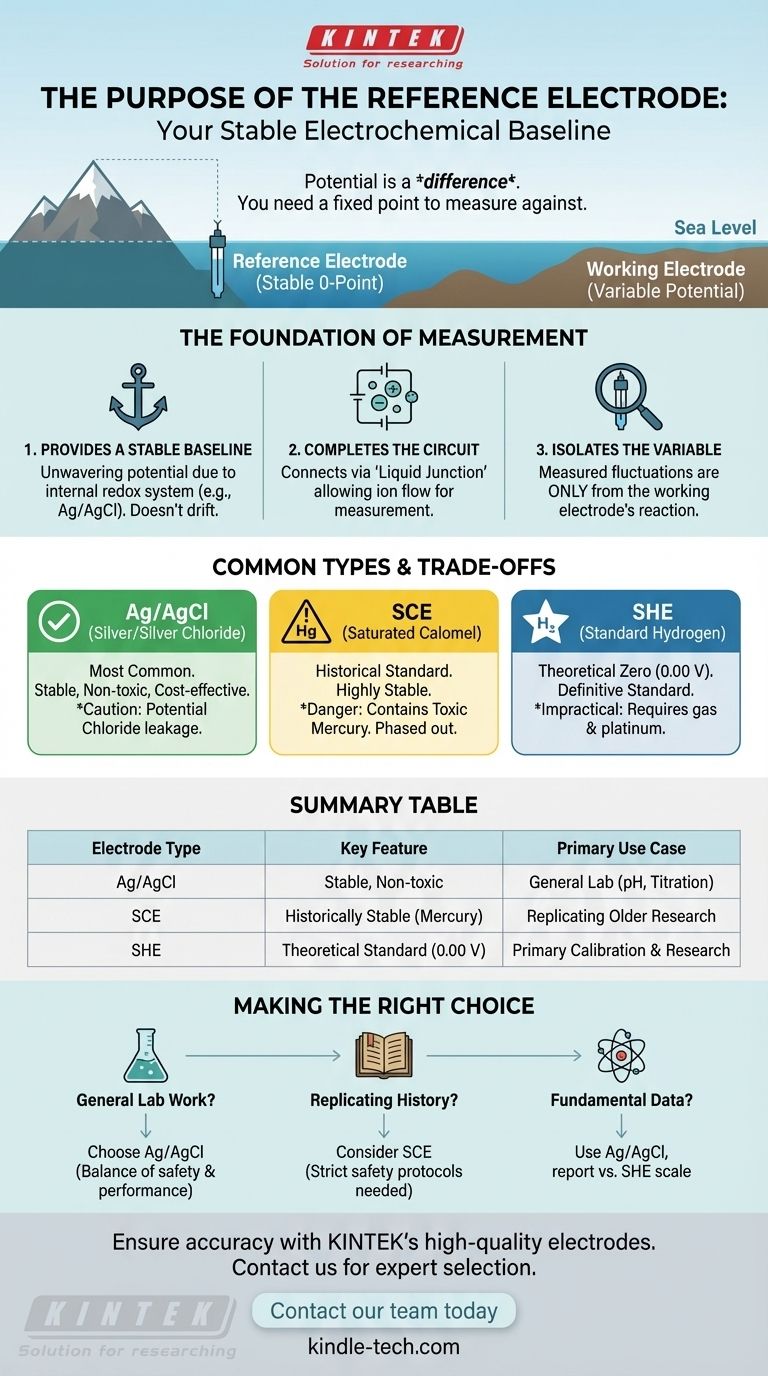
In any electrochemical system, the purpose of a reference electrode is to provide a stable, constant, and well-known potential. It acts as a fixed baseline, or zero point, against which the potential of a second electrode—the working electrode—can be measured. By completing the electrical circuit, it makes a meaningful measurement possible.
You cannot measure the potential of a single electrode in isolation; potential is always a difference between two points. The reference electrode provides a reliable, unchanging half of the system, ensuring that any change you measure is due solely to the chemical reaction you are studying at the working electrode.

The Foundation of Electrochemical Measurement
To understand the reference electrode, you must first understand that every electrochemical measurement requires two electrodes to form a complete cell. The reference electrode is one of these essential components.
Providing a Stable Baseline
Potential is a relative value, much like the height of a mountain is measured relative to sea level. The reference electrode acts as the unwavering "sea level" for your measurement.
Its internal chemistry is carefully designed using a redox system (like silver and silver chloride) with saturated or buffered solutions. This ensures its own potential does not drift or change during the experiment.
Completing the Electrical Circuit
A theoretical baseline is useless without a physical connection. The reference electrode makes contact with the sample solution through a component called a liquid junction.
This junction allows ions to flow, completing the electrical circuit between the reference and working electrodes. Without this connection, no measurement could be taken.
Isolating the Variable of Interest
By providing a constant potential, the reference electrode allows the total measured potential of the cell to reflect only what is happening at the working electrode.
Any fluctuation detected is therefore directly attributable to the concentration of the analyte or the reaction being studied, which is the entire goal of the measurement.
Common Types and Their Trade-offs
While all reference electrodes serve the same purpose, they are not created equal. The choice of electrode involves practical trade-offs in stability, cost, and safety.
Silver/Silver Chloride (Ag/AgCl)
This is the most widely used reference electrode today. It is known for being relatively inexpensive, highly stable, and non-toxic.
Its primary drawback is the potential for the internal salt bridge solution (potassium chloride) to leak into the sample, which can be an issue in applications sensitive to chloride ions.
Saturated Calomel Electrode (SCE)
The SCE is a historically significant electrode renowned for its exceptional stability and reliability. It was once a laboratory standard.
However, it contains mercury, which is highly toxic. Due to safety concerns and disposal challenges, its use has been largely phased out in favor of the Ag/AgCl electrode.
Standard Hydrogen Electrode (SHE)
The SHE is the definitive, theoretical reference against which all other electrode potentials are defined. Its potential is set at exactly 0.00 volts by convention.
Despite its importance as a standard, the SHE is extremely impractical for daily use. It requires a constant supply of flammable hydrogen gas and a specially prepared platinum electrode, making it suitable only for primary calibration and research.
Making the Right Choice for Your Goal
Your choice of reference electrode is typically guided by your application's requirements for compatibility, precision, and safety.
- If your primary focus is general lab work (e.g., pH, titration): The Silver/Silver Chloride (Ag/AgCl) electrode is the standard choice for its excellent balance of performance, safety, and cost.
- If your primary focus is replicating older research: You may need to use a Saturated Calomel Electrode (SCE) to maintain consistency with historical data, but you must adhere to strict safety protocols.
- If your primary focus is establishing fundamental electrochemical data: You will use a practical electrode like Ag/AgCl but report your results relative to the Standard Hydrogen Electrode (SHE) scale for universal comparison.
Ultimately, the reference electrode is a simple but critical tool that removes ambiguity, allowing you to focus on the chemistry that matters.
Summary Table:
| Electrode Type | Key Feature | Primary Use Case |
|---|---|---|
| Silver/Silver Chloride (Ag/AgCl) | Stable, non-toxic, cost-effective | General lab work (pH, titration) |
| Saturated Calomel (SCE) | Historically stable, contains mercury | Replicating older research (with safety protocols) |
| Standard Hydrogen (SHE) | Theoretical standard (0.00 V) | Primary calibration & fundamental research |
Ensure the accuracy and reliability of your electrochemical experiments with the right reference electrode.
KINTEK specializes in high-quality lab equipment and consumables, including a range of reliable reference electrodes tailored for laboratory needs. Our experts can help you select the ideal electrode for your specific application, ensuring precise and stable measurements.
Contact our team today to discuss your requirements and enhance your lab's analytical capabilities!
Visual Guide
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
People Also Ask
- What are the post-treatment procedures after using a copper sulfate reference electrode? Essential Steps for Accuracy & Longevity
- Is there a difference in performance between wood plug and ceramic core copper sulfate electrodes? Speed vs. Durability Explained
- What precautions should be taken when handling and using a copper sulfate reference electrode? Ensure Accurate Electrochemical Measurements
- What are the pre-treatment steps before using a portable copper sulfate reference electrode? Ensure Accurate Corrosion Potential Measurements
- How should a copper sulfate reference electrode be maintained? Ensure Accurate Electrochemical Measurements



















