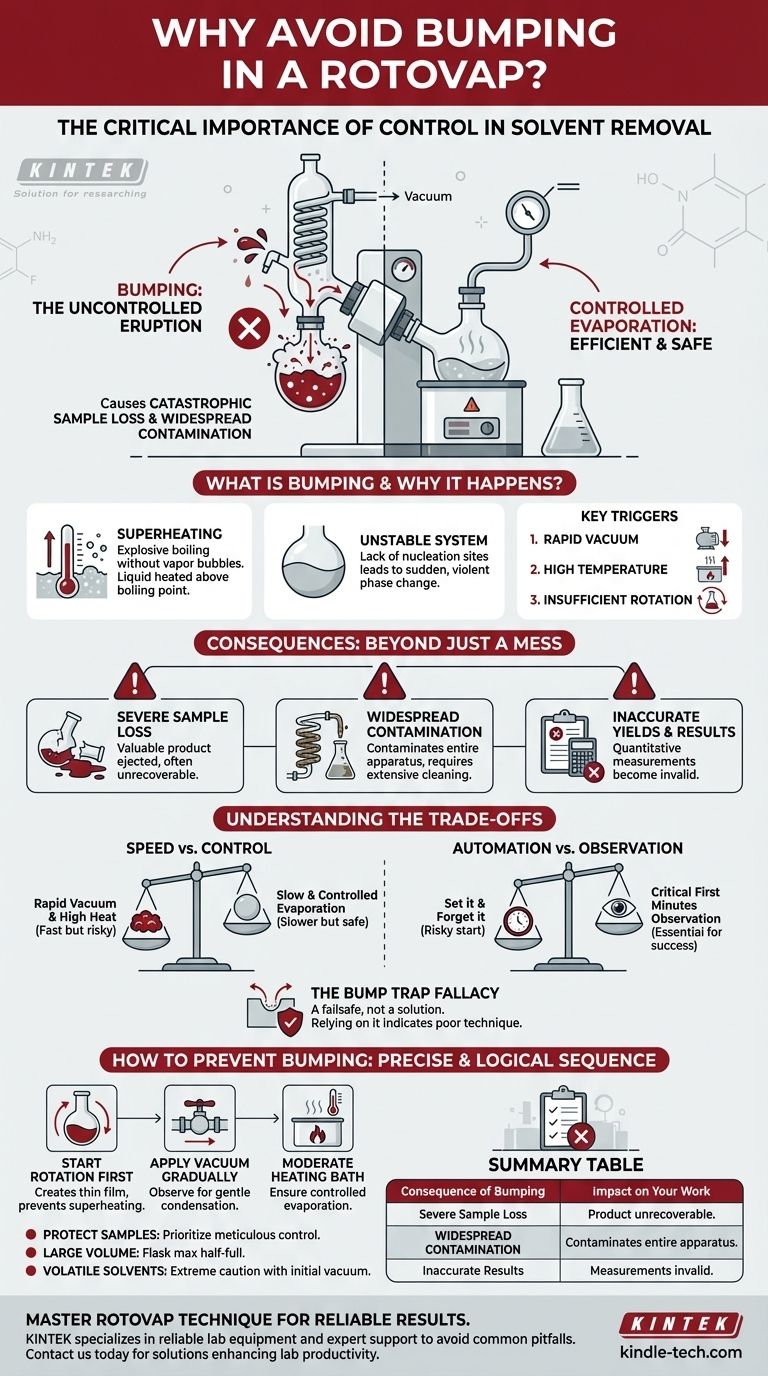
In short, you must avoid bumping in a rotovap because it causes catastrophic sample loss and contaminates the entire apparatus. Bumping is the sudden, violent eruption of your solution, which ejects your valuable product from the evaporating flask and spreads it throughout the condenser, receiving flask, and even the vacuum system.
The core issue is one of control. Bumping signifies a loss of control over the evaporation process, turning a gentle separation into a chaotic event that undermines the very purpose of using a rotovap: the careful and efficient removal of solvent.

What is "Bumping" and Why Does It Happen?
To prevent bumping, you first have to understand its physical cause. It is not simply vigorous boiling; it's a symptom of an unstable system.
Defining the Eruption
Bumping is the explosive boiling of a liquid that has been heated above its boiling point without forming vapor bubbles. This phenomenon is also known as superheating.
Instead of a steady stream of small bubbles, the energy builds up until a single, large bubble or a few massive bubbles form suddenly, ejecting the surrounding liquid with significant force.
The Physics of Uncontrolled Boiling
Normally, boiling begins at nucleation sites—tiny imperfections on the glass or dust particles where bubbles can form.
When a liquid is heated smoothly under vacuum without these sites (or with insufficient agitation), it can become superheated. Any slight disturbance can then trigger a sudden, violent phase change from liquid to gas, causing the "bump."
Key Triggers for Bumping
Bumping is almost always caused by improper technique. The three main triggers are:
- Vacuum applied too rapidly: This causes a sudden, dramatic drop in the solvent's boiling point, leading to instant, explosive boiling.
- Temperature is too high: If the heating bath is excessively hot relative to the system's pressure, the liquid can superheat easily.
- Insufficient or no rotation: Rotation continuously creates a thin film of the liquid on the flask wall. This increases the surface area for smooth evaporation and prevents localized superheating.
The Consequences: Beyond Just a Mess
Bumping isn't just an inconvenience; it can invalidate your entire experiment and create hours of cleanup work.
Severe Sample Loss
This is the most immediate and painful consequence. Your carefully synthesized or extracted product is physically thrown out of the flask. In many cases, this loss is unrecoverable.
Widespread Contamination
The bumped solution travels up into the vapor duct and washes down the condenser coils. It contaminates the receiving flask (distillate) and can even be sucked into the vacuum lines and pump.
This requires a complete disassembly and cleaning of the rotovap glassware, wasting significant time and introducing potential cross-contamination for future experiments.
Inaccurate Yields and Results
Even if you recover some material, you can no longer trust your results. Any quantitative measurement, such as calculating the final yield of a reaction, becomes meaningless after a significant bumping event.
Understanding the Trade-offs
Efficient rotovaping is a balancing act. Understanding the trade-offs helps you diagnose problems before they start.
Speed vs. Control
The most common mistake is sacrificing control for speed. Applying a very deep vacuum and high heat seems like the fastest way to remove solvent, but it is the primary recipe for bumping. A slow, controlled evaporation is almost always faster than a failed attempt that requires a complete reset.
Automation vs. Observation
Modern rotovaps have automated controls, but you should never "set it and forget it" at the beginning. The first few minutes are critical. You must observe the system as vacuum is applied to find the "sweet spot" where the solvent refluxes gently without the risk of bumping.
The Bump Trap Fallacy
Using a bump trap between the flask and the vapor duct is an essential safety measure, like a seatbelt. However, relying on it is a sign of poor technique. If you are regularly finding your product in the bump trap, you are not controlling the evaporation properly. It is a failsafe, not a solution.
How to Apply This to Your Project
The key to preventing bumping is to follow a precise and logical sequence of operations. Think of it as managing the relationship between rotation, pressure, and temperature.
- If your primary focus is protecting a valuable sample: Prioritize meticulous control by starting rotation first, then applying vacuum very gradually until you see gentle condensation, and only then lowering the flask into a moderately heated bath.
- If your primary focus is safely removing a large volume of solvent: Ensure your flask is never more than half-full to maximize surface area and prevent splashing, making controlled evaporation easier to manage.
- If you are working with a very volatile (low-boiling) solvent: Be extremely cautious with the initial vacuum, as the solvent may boil violently even at room temperature before you introduce any external heat.
Mastering these principles transforms the rotovap from a source of anxiety into a precise and reliable tool for your work.
Summary Table:
| Consequence of Bumping | Impact on Your Work |
|---|---|
| Severe Sample Loss | Product is ejected from the flask, often making it unrecoverable. |
| Widespread Contamination | Solution contaminates the condenser, receiving flask, and vacuum lines. |
| Inaccurate Results | Quantitative measurements like final yield become invalid and unreliable. |
Protect Your Valuable Samples and Ensure Reliable Results
Mastering rotovap technique is essential for efficient and safe solvent removal. KINTEK specializes in providing reliable lab equipment and expert support to help you avoid common pitfalls like bumping. Our range of rotary evaporators and consumables are designed for precise control, helping you achieve consistent, high-quality outcomes in your laboratory workflows.
Contact us today to discuss your specific application needs and discover how our solutions can enhance your lab's productivity and precision.
Visual Guide
Related Products
- Evaporation Crucible for Organic Matter
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
People Also Ask
- What are the 5 factors that affect the rate of evaporation? Master the Process for Your Lab
- What are the analytical used in laboratory? Choose the Right Tool for Your Lab's Needs
- How long does it take for THC to evaporate? The Real Science Behind Potency Loss
- What is the delta 20 rule of evaporation? Master Safe and Effective Spraying
- What factors affect evaporation and condensation? Master the Science of Water's Phase Changes



















