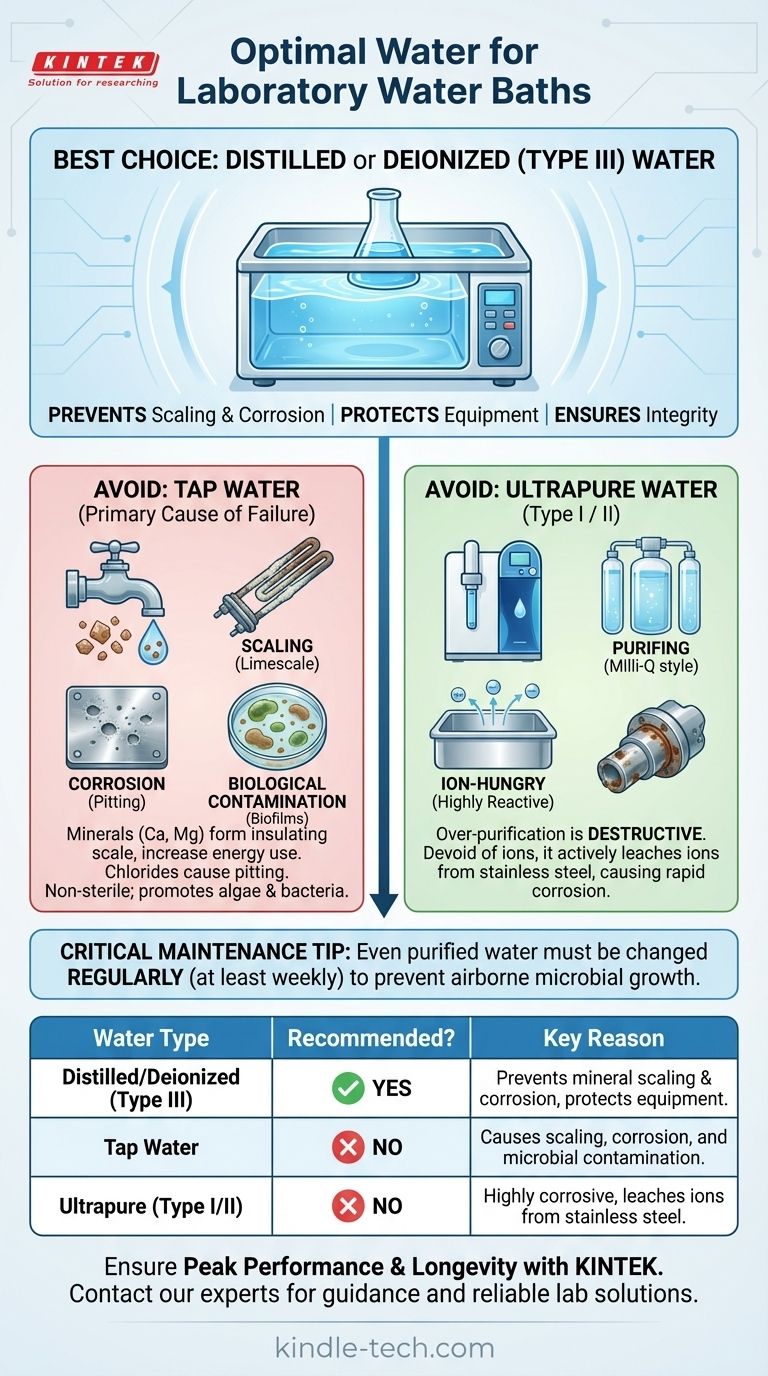
For optimal performance and equipment longevity, the recommended water for a laboratory water bath is distilled or deionized (Type III) water. Using standard tap water introduces minerals and contaminants that directly lead to scaling, corrosion, and potential contamination of your experiments.
The choice of water for your lab water bath is not a trivial detail; it's a fundamental aspect of equipment maintenance and experimental integrity. While tap water is convenient, its mineral content directly causes damage and contamination, making purified water the only reliable choice for professional laboratory work.

Why Tap Water Is the Primary Cause of Failure
Using tap water in a heated water bath may seem harmless, but it actively works against the equipment and your science. The issues stem from the dissolved solids and microorganisms it contains.
The Impact of Mineral Deposits (Scaling)
Tap water is rich in dissolved minerals, primarily calcium and magnesium carbonates. When the water is heated, these minerals precipitate out of the solution and form a hard, chalky layer known as limescale.
This scale builds up on the heating element and the tank's surface. Because scale is an excellent insulator, it forces the heating element to work harder and run hotter to achieve the target temperature, leading to increased energy consumption and eventual burnout.
The Risk of Corrosion
Beyond scaling minerals, tap water often contains chlorides and other ions that are highly corrosive to metals, including the stainless steel typically used in water bath tanks.
These ions attack the protective layer of the steel, leading to pitting corrosion. Over time, this damage can cause leaks and catastrophic failure of the unit.
The Threat of Biological Contamination
Tap water is not sterile. It contains a variety of microorganisms, such as bacteria and algae.
The warm, static environment of a water bath is a perfect incubator for these organisms. This results in the formation of biofilms, which can contaminate sensitive samples and create unpleasant odors.
Understanding the Right Water Choices
Purified water is essential, but it's important to use the correct grade. Not all pure water is suitable for this application.
Distilled Water (Type III)
Distillation is a process where water is boiled, and the resulting steam is condensed back into liquid form. This process effectively removes minerals, salts, and other non-volatile impurities.
Distilled water is an excellent and widely recommended choice for water baths, as it eliminates the root causes of scaling and corrosion.
Deionized Water (DI Water, Type III)
Deionization removes dissolved mineral ions by passing water through ion-exchange resins. For the purposes of a water bath, deionized (DI) water is functionally equivalent to distilled water.
It lacks the mineral content that causes limescale, making it a perfectly suitable and safe option for protecting your equipment.
Common Pitfalls and Trade-offs to Avoid
Choosing the right water involves understanding not just what to use, but also what to avoid. Certain common practices and assumptions can lead to unexpected problems.
The False Economy of Tap Water
The most significant trade-off is short-term convenience versus long-term cost. While tap water is essentially free, the cost of premature equipment failure, failed experiments, and increased energy usage far outweighs the minor expense and effort of using purified water.
The Danger of Over-Purification
It is a critical mistake to assume that "purer is better." Using ultrapure water (Type I or Type II), such as that from a Milli-Q system, will damage your water bath.
This water is so devoid of ions that it becomes highly reactive, actively leaching ions from the stainless steel tank and components. This "ion-hungry" nature causes rapid corrosion and is far more destructive than tap water.
The Need for Consistent Maintenance
Using purified water does not eliminate the need for maintenance. Water should still be changed regularly—at least weekly—to prevent the growth of airborne microorganisms that land in the bath. Using a lab-grade algaecide or water bath treatment can help, but it is not a substitute for regular cleaning.
Making the Right Choice for Your Lab
Your decision should be based on a commitment to reliable results and responsible equipment stewardship.
- If your primary focus is equipment longevity and reliability: Always use distilled or deionized (Type III) water and ensure it is changed at least weekly.
- If you are running highly sensitive biological or chemical experiments: Use fresh, high-quality distilled or deionized water for every critical run to eliminate any risk of microbial or chemical cross-contamination.
- If you must use tap water in an emergency: Do so only for a short time and plan to thoroughly descale and clean the unit immediately afterward to mitigate the inevitable mineral buildup.
Ultimately, treating your water bath with the correct type of water is the single most effective way to ensure its accuracy, reliability, and longevity.
Summary Table:
| Water Type | Recommended for Water Bath? | Key Reason |
|---|---|---|
| Distilled/Deionized (Type III) | Yes | Prevents mineral scaling and corrosion, protects equipment |
| Tap Water | No | Causes scaling, corrosion, and microbial contamination |
| Ultrapure (Type I/II) | No | Highly corrosive, leaches ions from stainless steel |
Ensure your laboratory water baths operate at peak performance and longevity. Using the correct water is fundamental to protecting your valuable equipment and ensuring the integrity of your experiments. KINTEK specializes in providing reliable lab equipment and consumables, including guidance on proper maintenance practices. Don't let improper water choice compromise your results—contact our experts today to discuss your laboratory needs and how we can support your success.
Visual Guide
Related Products
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
- 50L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 20L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 30L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 10L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
People Also Ask
- What are the 3 main substances used for biomass fuel? Unlock Sustainable Energy from Organic Matter
- Why is KBr and NaCl used in IR spectroscopy? Achieve Clear, Accurate Sample Analysis
- What is an example of a magnetron sputtering? Creating High-Performance Coatings for Eyeglasses & Electronics
- What are the advantages and limitations of heat treatment process? Unlock Material Performance
- What is the simulated annealing method? A Powerful Optimization Algorithm Explained
- What are the raw materials for bio-oil? A Guide to Selecting the Best Biomass Feedstock
- What are the pros and cons of biomass? Weighing Renewable Energy Against Environmental Impact
- What is the process of sputtering? A Step-by-Step Guide to Thin Film Deposition


















