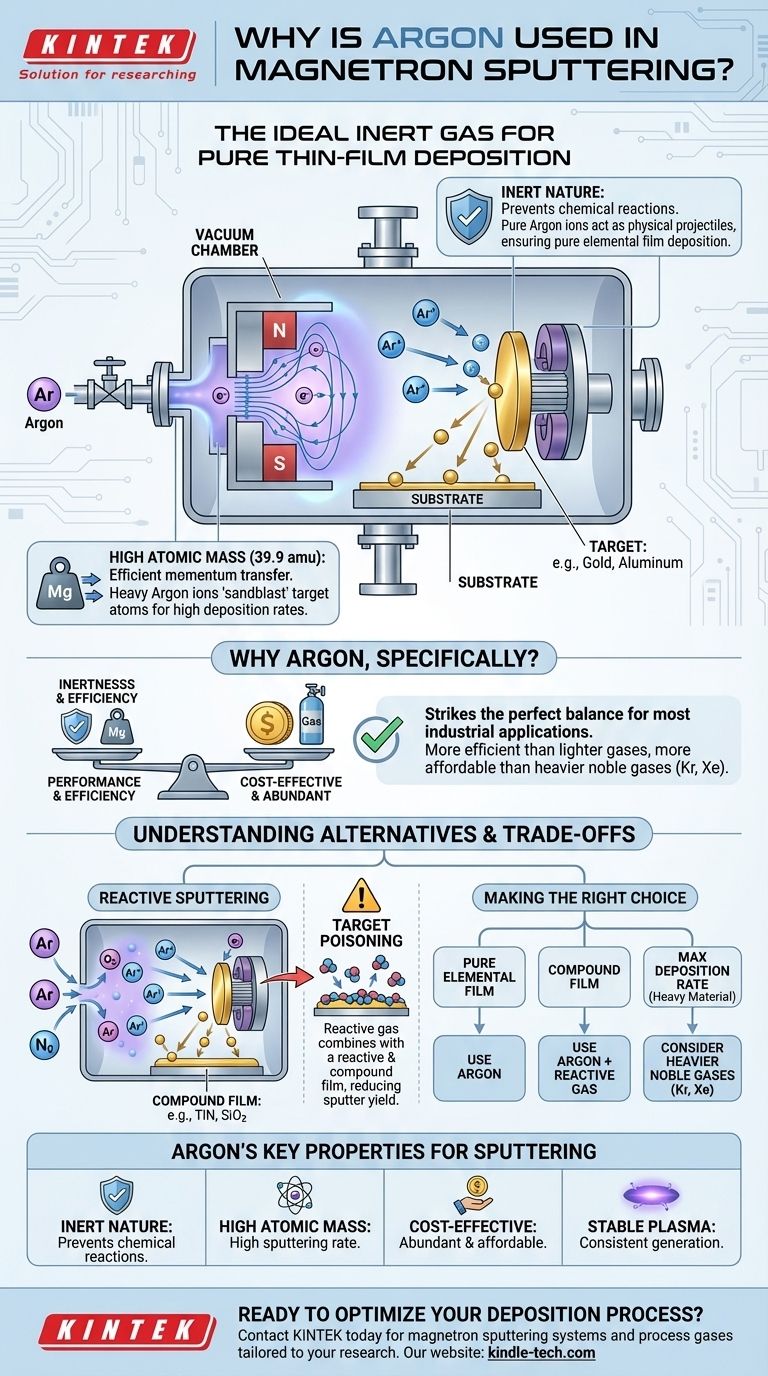
At its core, argon is used in magnetron sputtering because it is an inert gas with a relatively high atomic mass. Its inert nature prevents it from chemically reacting with the target material, ensuring a pure film is deposited, while its mass allows its ions to effectively "sandblast" atoms from the target surface with high efficiency.
The choice of gas in sputtering is not just about creating a plasma; it is a fundamental decision that dictates the physics of material ejection and the chemistry of the resulting film. Argon is the workhorse for purely physical deposition, but understanding why allows you to master more complex reactive processes.

The Core Role of Gas in Sputtering
To understand argon's prevalence, we must first look at the role any gas plays in the sputtering process. The gas is the medium that is transformed into a tool for material ejection.
Creating the Plasma
The process begins by introducing a low-pressure gas, like argon, into a vacuum chamber. A strong electric field is then applied.
This field energizes free electrons, which collide with the neutral argon atoms. These collisions are energetic enough to knock an electron off the argon atoms, creating positively charged argon ions (Ar+) and more free electrons. This self-sustaining cloud of ions and electrons is the plasma.
The Importance of Being Inert
Argon is a noble gas, meaning it is chemically inert. It does not readily form chemical bonds with other elements.
This property is critical for physical vapor deposition (PVD). The goal is to physically transport material from the target to the substrate without altering its chemistry. Using an inert gas ensures the argon ions simply act as physical projectiles, preventing unwanted chemical reactions on the target or the growing film.
The Impact of Mass
Sputtering is a momentum transfer process. Think of it as a microscopic game of billiards. When a high-energy argon ion strikes the target, it transfers its momentum to the target atoms.
A heavier ion carries more momentum than a lighter one at the same energy. Therefore, a heavier ion like argon is more effective at ejecting, or sputtering, target atoms. This results in a higher deposition rate, making the process more efficient.
Why Argon, Specifically?
While other noble gases exist, argon hits the ideal balance of performance, cost, and practicality for the vast majority of sputtering applications.
The Balance of Performance and Cost
Argon provides an excellent combination of being inert and having a sufficient atomic mass (39.9 amu) for efficient sputtering of most materials.
While heavier noble gases like Krypton (Kr) or Xenon (Xe) would yield even higher sputtering rates due to their greater mass, they are significantly rarer and more expensive. Argon’s abundance in the atmosphere (nearly 1%) makes it far more cost-effective for industrial use.
Stable Plasma Generation
Argon has an ionization potential that is well-suited for creating and sustaining a stable plasma under typical vacuum and power conditions used in magnetron systems. It strikes a balance, being easy enough to ionize without becoming too unstable.
Understanding the Trade-offs and Alternatives
While argon is the default, it is not the only option. Understanding the alternatives reveals the deeper strategic goals of thin-film deposition.
When Argon Isn't Enough: Reactive Sputtering
Sometimes, the goal is not to deposit a pure material but to create a specific chemical compound, such as an oxide or nitride. This is called reactive sputtering.
In this process, a reactive gas like oxygen (O2) or nitrogen (N2) is intentionally introduced into the chamber along with the argon. The argon ions still do the primary work of sputtering the metallic target, but the reactive gas combines with the sputtered atoms as they travel to and land on the substrate, forming a compound film like titanium nitride (TiN) or silicon dioxide (SiO2).
The Pitfall: Target Poisoning
A major challenge in reactive sputtering is target poisoning. This occurs when the reactive gas begins to form a compound layer (e.g., a nitride or oxide) directly on the surface of the target itself.
This "poisoned" layer often has a much lower sputter yield than the pure metal target. As a result, the deposition rate can plummet dramatically, making the process unstable and difficult to control. Managing the partial pressure of the reactive gas is critical to avoid this effect.
How the Magnetron Amplifies the Process
The "magnetron" in magnetron sputtering is a crucial enhancement that makes the use of argon so effective.
Concentrating the Plasma
A magnetron uses a configuration of powerful magnets placed behind the sputtering target. This magnetic field traps the highly mobile electrons from the plasma, forcing them into a spiral path directly in front of the target surface.
Increasing Ionization Efficiency
By trapping electrons near the target, the magnetron dramatically increases the probability that these electrons will collide with and ionize neutral argon atoms.
This creates a dense, intense plasma concentrated precisely where it is needed most. This allows for very high sputtering rates at much lower operating pressures compared to non-magnetron sputtering, resulting in higher quality films.
Making the Right Choice for Your Goal
Your choice of gas is determined entirely by the properties you need in your final thin film.
- If your primary focus is depositing a pure elemental film (e.g., pure gold or aluminum): Argon is the definitive and most cost-effective choice for its inertness and efficiency.
- If your primary focus is creating a hard, ceramic, or optical compound film (e.g., TiN, Al2O3): You must use a carefully controlled mixture of argon and a reactive gas like nitrogen or oxygen.
- If your primary focus is maximizing the deposition rate of a very heavy or difficult-to-sputter material: You might consider a heavier, more expensive noble gas like krypton or xenon, if the budget permits and the efficiency gain is justified.
Ultimately, selecting the right process gas is about controlling the fundamental physics and chemistry within the plasma to achieve the precise film properties you require.
Summary Table:
| Property | Why It Matters for Sputtering |
|---|---|
| Inert Nature | Prevents chemical reactions, ensuring a pure film is deposited without contamination. |
| High Atomic Mass | Enables efficient momentum transfer for high sputtering rates and faster deposition. |
| Cost-Effectiveness | Abundant and affordable, making it ideal for industrial and research applications. |
| Stable Plasma | Easy to ionize, allowing for consistent and reliable plasma generation in the chamber. |
Ready to optimize your thin-film deposition process? The choice of sputtering gas is critical to achieving the precise film properties your research demands. At KINTEK, we specialize in providing high-quality lab equipment and consumables, including magnetron sputtering systems and process gases tailored to your specific application—whether you need pure argon for elemental films or guidance on reactive gas mixtures for compound films. Let our experts help you enhance your lab's efficiency and film quality. Contact KINTEK today to discuss your laboratory needs and discover the right solution for you!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Molybdenum Tungsten Tantalum Special Shape Evaporation Boat
People Also Ask
- What is the function of a laboratory forced-air drying oven? Optimize Prosopis Juliflora Moisture Analysis
- What is the basic instrument for IR spectrometry? FT-IR Spectrometers for Modern Chemical Analysis
- What is the difference between DC sputtering and RF sputtering? Choose the Right Technique for Your Material
- What is the trend in Synthetic Diamonds? Exponential Growth Reshaping the Gemstone Market
- What is the role of an ultrasonic cleaner during magnesium alloy coating? Ensure Superior Adhesion and Surface Purity
- Can aluminum be sintered? Overcome the Oxide Barrier for Complex, Lightweight Parts
- What are researchers trying to do to make biofuels cheaper? Unlock Affordable, Sustainable Energy with Advanced Bioengineering
- How does a high-precision temperature control heating system ensure accurate corrosion kinetics? Expert Lab Solutions



















