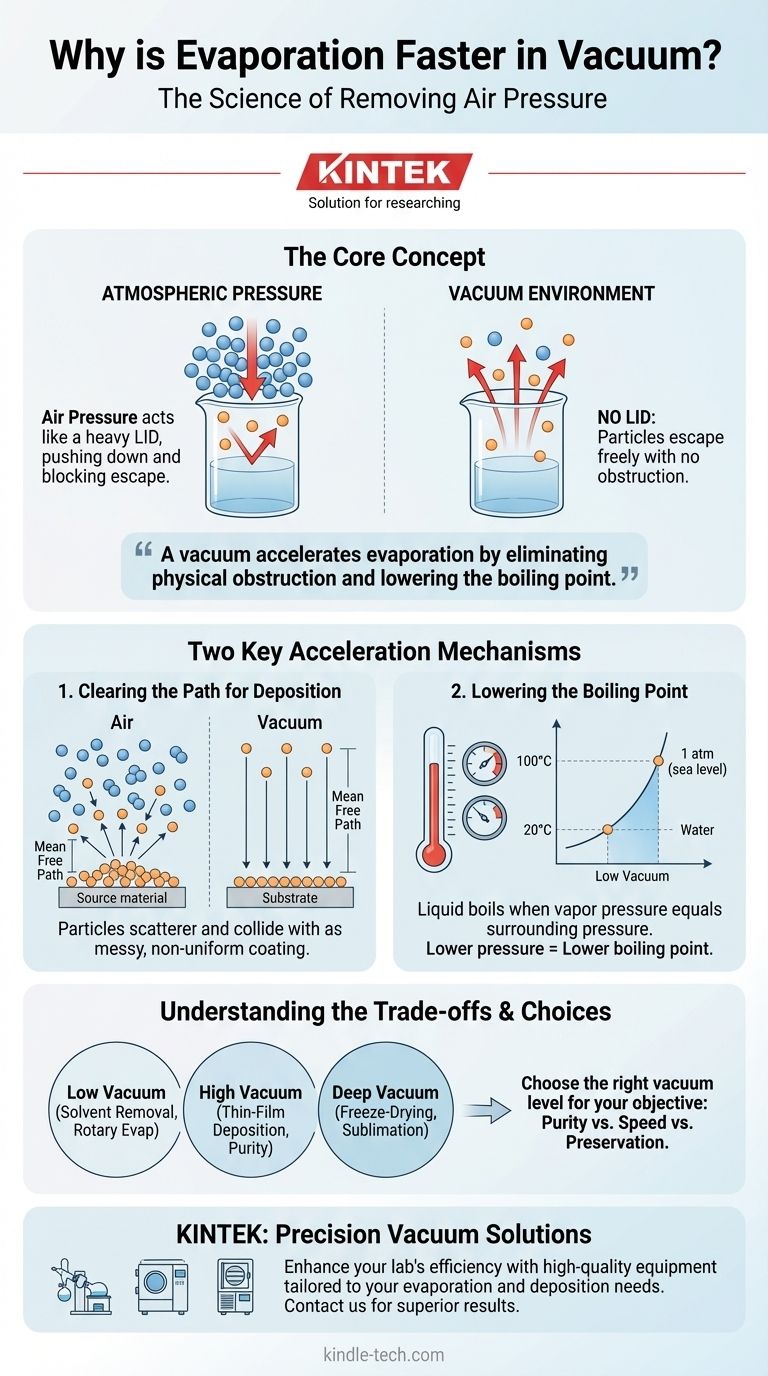
Fundamentally, evaporation is faster in a vacuum because there is no air pressure pushing down on the liquid's surface. Without the physical barrier of air molecules, particles can escape the liquid phase far more easily and at lower temperatures. This creates a clear, unobstructed path for vapor to travel, dramatically increasing the rate of the phase change from liquid to gas.
A vacuum accelerates evaporation by addressing two fundamental barriers: it eliminates the physical obstruction of air molecules and lowers the liquid's boiling point. This doesn't just make the process faster; it makes it more efficient and controllable, especially in technical applications.

The Physics of Pressure and Evaporation
To understand why a vacuum has such a profound effect, we must first revisit the basic principles of evaporation and pressure.
What is Evaporation?
Evaporation is the process where molecules at the surface of a liquid gain enough kinetic energy to overcome intermolecular forces and escape into the gaseous phase. This is a constant process occurring at any temperature above absolute zero.
The Role of Atmospheric Pressure
Under normal conditions, the liquid's surface is constantly being bombarded by molecules from the air above it. This atmospheric pressure acts like a physical lid, pushing down on the liquid and making it more difficult for surface molecules to escape.
An escaping molecule is likely to collide with an air molecule (like nitrogen or oxygen) and be knocked back into the liquid.
How a Vacuum Changes the Equation
Creating a vacuum means systematically removing gas molecules from a closed system. As the pressure drops, the "lid" of air is effectively lifted.
With far fewer gas molecules above the surface, there is significantly less opposition. Liquid molecules can escape into the gas phase much more freely, and the chance of a collision that would send them back is drastically reduced.
Two Key Mechanisms of Acceleration
Removing air pressure speeds up evaporation through two distinct but related physical mechanisms.
Mechanism 1: Clearing the Path for Deposition
In technical applications like vacuum deposition, the goal isn't just to evaporate a material but to have it travel to and coat a target (a substrate). A vacuum is essential for this.
Without a vacuum, evaporated particles would collide with billions of air molecules, scattering in random directions and never reaching their target in a controlled way.
In a high vacuum, the mean free path—the average distance a particle can travel before colliding with another—becomes very long. This allows evaporated atoms to travel in a straight line directly from the source to the substrate, ensuring a pure and uniform coating.
Mechanism 2: Lowering the Boiling Point
Boiling is simply a rapid, bulk form of evaporation. A liquid boils when its vapor pressure equals the pressure of the surrounding environment.
At sea level, water boils at 100°C (212°F) because that is the temperature at which its vapor pressure equals standard atmospheric pressure.
By lowering the pressure in a vacuum chamber, you lower the threshold the vapor pressure needs to reach. This means the liquid will boil at a much lower temperature, leading to extremely rapid evaporation without needing to add excessive heat. This is the core principle behind a rotary evaporator.
Understanding the Trade-offs and Practical Limits
While a vacuum is a powerful tool, its application is not without practical considerations and limitations.
The Law of Diminishing Returns
Achieving a "perfect" vacuum is impossible. Each successive drop in pressure requires exponentially more energy and more sophisticated equipment.
For many processes, a "low" vacuum is sufficient to significantly lower the boiling point. The expense of achieving an "ultra-high" vacuum is only justified for sensitive applications like thin-film deposition where particle purity is paramount.
Process Control Challenges
Lowering the pressure too quickly can cause violent boiling, a phenomenon known as bumping. This can lead to sample loss and contamination of the vacuum system.
Effective vacuum evaporation requires a careful balance between pressure and temperature control to ensure a smooth, manageable process.
Equipment and Energy Costs
High-vacuum pumps and chambers are expensive to purchase, operate, and maintain. The energy required to create and hold a deep vacuum is a significant factor in industrial processes, representing a direct operational cost.
Making the Right Choice for Your Goal
The level of vacuum you need is dictated entirely by your objective.
- If your primary focus is material deposition (e.g., thin films): Your goal is purity and a long mean free path, so a high or ultra-high vacuum is non-negotiable.
- If your primary focus is solvent removal (e.g., rotary evaporation): Your goal is speed at a low temperature, making a low vacuum perfectly sufficient to drastically lower the solvent's boiling point.
- If your primary focus is dehydration (e.g., freeze-drying): You need a deep vacuum to allow sublimation (solid to gas) to occur efficiently, preserving the material's structure without damaging heat.
Ultimately, using a vacuum is about creating an ideal environment to control a physical process with precision.
Summary Table:
| Vacuum Level | Key Mechanism | Common Applications |
|---|---|---|
| Low Vacuum | Lowers boiling point for rapid solvent removal | Rotary Evaporation, Concentration |
| High/Ultra-High Vacuum | Creates long mean free path for pure material travel | Thin-Film Deposition, Coating |
| Deep Vacuum | Enables sublimation (solid to gas) without heat damage | Freeze-Drying, Lyophilization |
Ready to Enhance Your Lab's Efficiency with Precision Vacuum Equipment?
At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your specific evaporation and deposition needs. Whether you're working on solvent removal, thin-film coating, or freeze-drying, our vacuum solutions offer the precise control, reliability, and efficiency your laboratory requires.
Let us help you achieve superior results with equipment designed for optimal performance. Contact us today to discuss your application and discover how KINTEK can support your laboratory's success!
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Lab-Scale Vacuum Induction Melting Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
People Also Ask
- What is the voltage of e-beam evaporation? Achieve Precise Thin-Film Deposition
- How is a thin film prepared by thermal evaporation? Master the Vacuum Deposition Process
- What is thermal evaporation of thin film deposition? A Simple Guide to PVD Coating
- What are the advantages and disadvantages of evaporative deposition? Achieve High-Purity Thin Films
- What is the use of electron beam evaporation? Achieve High-Purity Thin Films for Demanding Applications
- What is the difference between sputtering and evaporation techniques? A Guide to Choosing the Right PVD Method
- What are the advantages of electron beam evaporation? Achieve High-Purity, High-Rate Thin Films
- What is the process of thermal evaporation deposition? A Simple Guide to Thin Film Coating



















