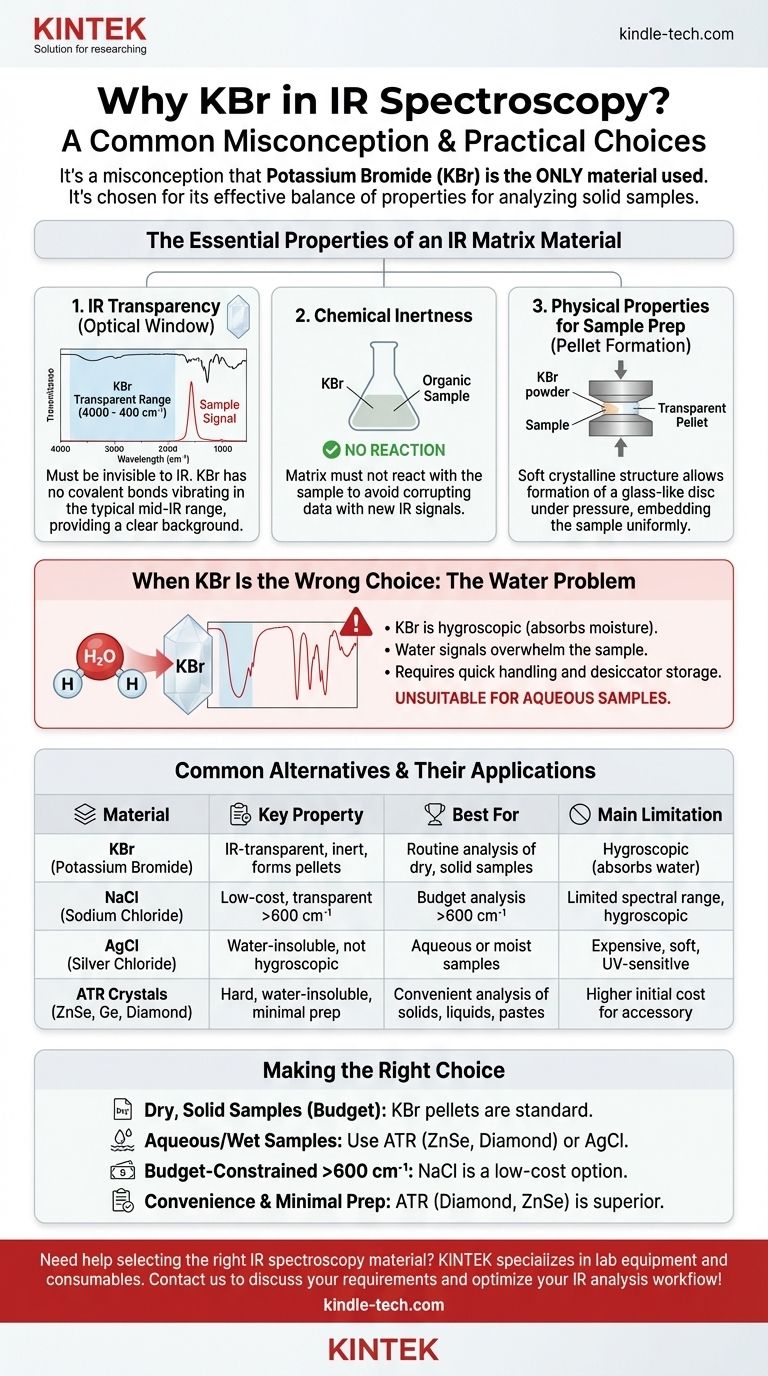
While Potassium Bromide (KBr) is exceptionally common in IR spectroscopy, it is a significant misconception that it is the only material used. Its prevalence is due to a highly effective combination of properties: it is transparent to infrared radiation across the most commonly used frequency range, it is chemically inert for most organic samples, and its soft crystalline structure is ideal for forming transparent pellets under pressure. This makes it a versatile and cost-effective choice for analyzing solid samples.
The core principle in selecting a material for IR spectroscopy is not loyalty to a single compound, but the fundamental need for optical transparency in the infrared region of interest. The chosen substance, whether a pellet matrix or a window, must not have its own absorption bands that would obscure the spectral features of the sample being analyzed.

The Essential Properties of an IR Matrix Material
To understand why KBr is such a popular choice, we must first define the ideal characteristics of a material used to hold a sample for IR analysis.
The Critical Need for IR Transparency
The primary function of a material like KBr is to act as a solid-state solvent or a "window" for the sample. It must be invisible to the infrared spectrometer.
KBr has no covalent bonds that vibrate within the typical mid-infrared range (4000 cm⁻¹ to 400 cm⁻¹). Its ionic lattice vibrations occur at very low frequencies, far below the standard analytical region. This makes it an almost perfectly clear background against which the sample's molecular vibrations can be measured accurately.
Chemical Inertness
The matrix material must not react with the sample. Any chemical reaction would create new substances with their own IR signals, corrupting the data.
Alkali halides, including KBr and NaCl, are generally unreactive with most organic and many inorganic compounds, ensuring the integrity of the sample during analysis.
Physical Properties for Sample Preparation
For solid samples, the most common preparation method is the KBr pellet technique. This is where KBr's physical properties are a distinct advantage.
KBr is a relatively soft, crystalline salt. When finely ground with a sample and subjected to high pressure (several tons), the KBr powder flows and fuses, forming a solid, glass-like disc that is transparent to IR light. This process embeds the sample in a uniform matrix, minimizing light scattering and allowing for high-quality spectral data.
When KBr Is the Wrong Choice (And What to Use Instead)
Despite its strengths, KBr is not a universal solution. Its primary weakness dictates the use of several common alternatives.
The Water Problem: KBr's Main Weakness
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong and broad absorption bands in the IR spectrum, which can easily overwhelm the signals from the actual sample.
This requires KBr pellets to be prepared and handled quickly, and they must be stored in a desiccator to prevent water contamination. Critically, KBr is completely unsuitable for analyzing samples that contain water.
Common Alternatives for Different Needs
The limitations of KBr have led to the use of other materials tailored for specific applications.
- Sodium Chloride (NaCl): Like KBr, NaCl is an inexpensive alkali halide transparent in much of the mid-IR range. However, its usable range ends around 600 cm⁻¹, cutting off the lower-frequency "fingerprint" region where KBr is still transparent. It is also hygroscopic.
- Silver Chloride (AgCl): A key advantage of AgCl is that it is insoluble in water and not hygroscopic. This makes it a suitable choice for running spectra of aqueous solutions or moist samples. However, it is more expensive, softer, and can be discolored by UV light.
- ATR Accessories (ZnSe, Ge, Diamond): Modern spectroscopy often favors Attenuated Total Reflectance (ATR) over pellet-making. This technique requires only placing the sample in direct contact with a high-refractive-index crystal. Materials like Zinc Selenide (ZnSe), Germanium (Ge), and especially Diamond are used for these crystals because they are extremely hard, chemically inert, and water-insoluble, allowing for rapid analysis of solids, liquids, and pastes with almost no sample preparation.
Making the Right Choice for Your Analysis
Choosing the correct infrared-transparent material is a practical decision based on your sample, budget, and the spectral region you need to investigate.
- If your primary focus is routine analysis of dry, solid samples: KBr is the cost-effective industry standard for creating high-quality transmission pellets.
- If your primary focus is analyzing aqueous solutions or wet samples: Avoid hygroscopic salts like KBr and use an ATR accessory with a ZnSe or diamond crystal.
- If your primary focus is budget-constrained analysis above 600 cm⁻¹: NaCl is a viable, lower-cost alternative to KBr, but be aware of its more limited spectral range.
- If your primary focus is convenience and minimal sample prep: The ATR technique, using a crystal like diamond or ZnSe, is the superior modern choice for a wide variety of sample types.
Ultimately, the goal is to select a material whose optical and chemical properties are perfectly matched to the specific requirements of your sample and analysis.
Summary Table:
| Material | Key Property | Best For | Main Limitation |
|---|---|---|---|
| KBr (Potassium Bromide) | IR-transparent, chemically inert, forms pellets | Routine analysis of dry, solid samples | Hygroscopic (absorbs water) |
| NaCl (Sodium Chloride) | Low-cost, IR-transparent above 600 cm⁻¹ | Budget analysis above 600 cm⁻¹ | Limited spectral range, hygroscopic |
| AgCl (Silver Chloride) | Water-insoluble, not hygroscopic | Aqueous or moist samples | Expensive, soft, UV-sensitive |
| ATR Crystals (ZnSe, Ge, Diamond) | Hard, water-insoluble, minimal prep | Convenient analysis of solids, liquids, pastes | Higher initial cost for accessory |
Need help selecting the right IR spectroscopy material for your specific application? KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can guide you to the ideal solution—whether you require KBr pellets, ATR accessories, or alternative materials—to ensure accurate, high-quality spectral data for your samples. Contact us today to discuss your requirements and optimize your IR analysis workflow!
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What is the role of a constant temperature shaking incubator in the immobilization of cadmium ions by SRB?
- What are the advantages of using Extremely fast Joule Heating (EJH) equipment? Precision in Thin Film Synthesis
- What types of biological materials can be safely stored at -70C? A Guide to Long-Term Sample Preservation
- How does temperature affect melting? Master Precise Control for Material Integrity
- Why magnets are placed behind the target in sputtering? To Trap Electrons for Faster, Purer Coatings
- What are the products of sludge pyrolysis? Transform Waste into Biochar, Bio-oil, and Syngas
- What are the key issues in the synthesis of nanomaterials? Overcoming Size, Shape, and Purity Control Challenges
- What are the problems with pyrolysis oil? Key Challenges in Bio-Oil Stability and Cost



















