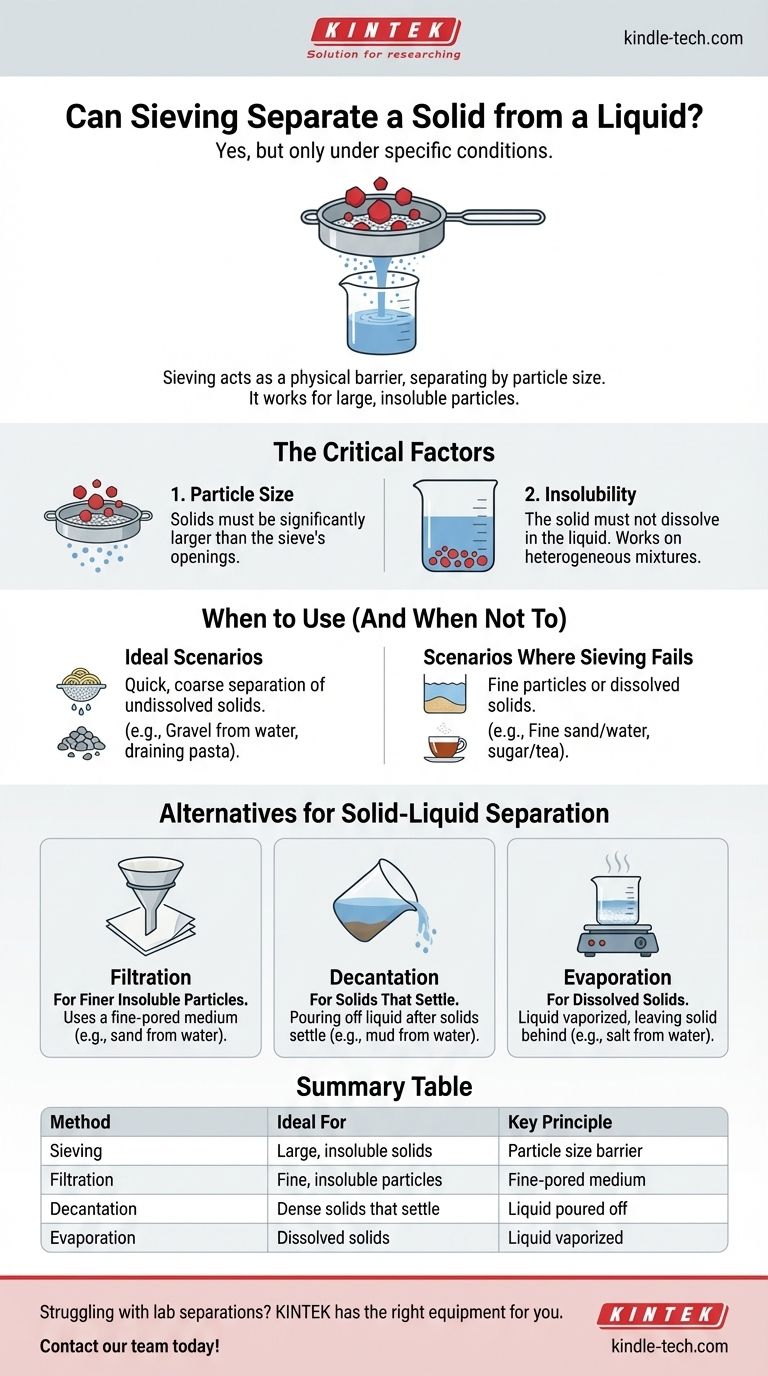
Yes, but only under specific conditions. Sieving can be used to separate a solid from a liquid, but only when the solid particles are insoluble and large enough to be caught by the openings in the sieve. This method works by acting as a physical barrier, allowing the liquid to pass through while retaining the larger solid components.
The core principle is simple: sieving separates mixtures based on particle size. It is the right tool for large, undissolved solids, but for fine particles or dissolved substances, you must turn to more precise methods like filtration or evaporation.

The Core Principle of Sieving
How Sieving Works
Sieving, often called sifting, is a mechanical separation process. It uses a mesh or screen with uniform openings.
When a mixture is poured onto the sieve, components smaller than the openings pass through, while components larger than the openings are retained. In a solid-liquid mixture, the liquid molecules easily pass through, along with any very fine suspended or dissolved solids.
The Critical Factor: Particle Size
The success of sieving depends entirely on a significant difference in size between the solid particles and the sieve's holes.
Think of draining cooked pasta in a colander. The pasta (large solid) is trapped, while the water (liquid) flows freely through. This is a perfect application of sieving.
The Second Critical Factor: Insolubility
Sieving only works on heterogeneous mixtures, where the solid does not dissolve in the liquid.
If you pour saltwater through a sieve, both the salt ions and the water molecules will pass through. Since the salt is dissolved, its individual particles are far too small to be caught. The mixture remains unchanged.
When to Use Sieving (And When Not To)
Ideal Scenarios for Sieving
Sieving is the ideal method for quick, coarse separations of undissolved solids.
Common examples include separating gravel from water, removing pulp from juice, or draining canned vegetables. The process is fast, simple, and requires minimal equipment.
Scenarios Where Sieving Fails
This method is ineffective for separating mixtures with very fine or dissolved solids.
Attempting to separate fine sand or silt from water with a standard kitchen sieve will fail because the sand particles are small enough to pass through the mesh along with the water. For a dissolved solid like sugar in tea, sieving is completely useless.
Understanding the Alternatives for Solid-Liquid Separation
If sieving isn't the right tool, another method will be. The correct choice depends on the properties of your mixture.
Filtration: For Finer Insoluble Particles
Filtration is essentially sieving on a microscopic level. It uses a filter medium (like paper or cloth) with pores far smaller than a sieve's mesh.
This is the correct technique for separating fine, insoluble particles, such as sand from water or coffee grounds from brewed coffee.
Decantation: For Solids That Settle
Decantation is used when a dense, insoluble solid settles at the bottom of a liquid over time, forming a sediment.
The process involves carefully pouring off the liquid, leaving the solid behind. It's a simple method for separating things like mud from water after the mud has settled.
Evaporation: For Dissolved Solids
When a solid is dissolved in a liquid, creating a solution, the only way to separate them is through evaporation.
By heating the solution, the liquid turns into a gas and evaporates, leaving the solid behind. This is the classic method for recovering salt from saltwater.
Choosing the Right Separation Method
Your choice of technique depends on the properties of your specific solid-liquid mixture.
- If your primary focus is a quick separation of large, undissolved solids: Sieving is your most efficient choice.
- If you are separating fine, undissolved particles for high purity: Use filtration for a more precise result.
- If the solid is completely dissolved in the liquid to form a solution: Evaporation is the only method that will recover the solid.
- If your insoluble solid is dense and settles quickly on its own: Decantation is a simple and effective option that requires no special equipment.
Understanding the nature of your mixture is the key to selecting the most effective separation technique.
Summary Table:
| Separation Method | Ideal For | Key Principle |
|---|---|---|
| Sieving | Large, insoluble solids (e.g., gravel from water) | Particle size difference; acts as a physical barrier |
| Filtration | Fine, insoluble particles (e.g., sand from water) | Uses a fine-pored medium to trap small particles |
| Evaporation | Dissolved solids in a solution (e.g., salt from water) | Liquid is vaporized, leaving the solid behind |
| Decantation | Dense solids that settle (e.g., mud from water) | Liquid is carefully poured off after settling |
Struggling to separate your lab mixtures efficiently? The right equipment is key to achieving pure, accurate results. KINTEK specializes in high-quality lab sieves, filtration systems, and evaporation equipment designed for reliability and precision. Whether you're processing samples or developing new methods, our experts can help you select the perfect tool for your specific solid-liquid separation needs.
Contact our team today to discuss your application and find the ideal solution for your laboratory!
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Laboratory Vibratory Sieve Shaker Machine for Dry and Wet Three-Dimensional Sieving
- Laboratory Vibratory Sieve Shaker Machine Slap Vibrating Sieve
- Vibratory Sieve Shaker Machine Dry Three-Dimensional Vibrating Sieve
- Laboratory Vortex Mixer Orbital Shaker Multifunctional Rotation Oscillation Mixer
People Also Ask
- Why is a standardized sieving system necessary for elephant grass research? Ensure Reliable Sample Consistency
- Why is a laboratory electromagnetic vibratory sieve shaker used? Optimize Walnut Shell Chemical Pretreatment
- How do high-precision sieving systems benefit zeolite preparation? Maximize Adsorption for Wastewater Treatment
- Why is powder classification using standard sieves essential for SHS reactions? Unlock Superior Nitriding Results
- Why use a vibratory sieve shaker for PET powder? Achieve Precise Particle Size Control for Chemical Research



















