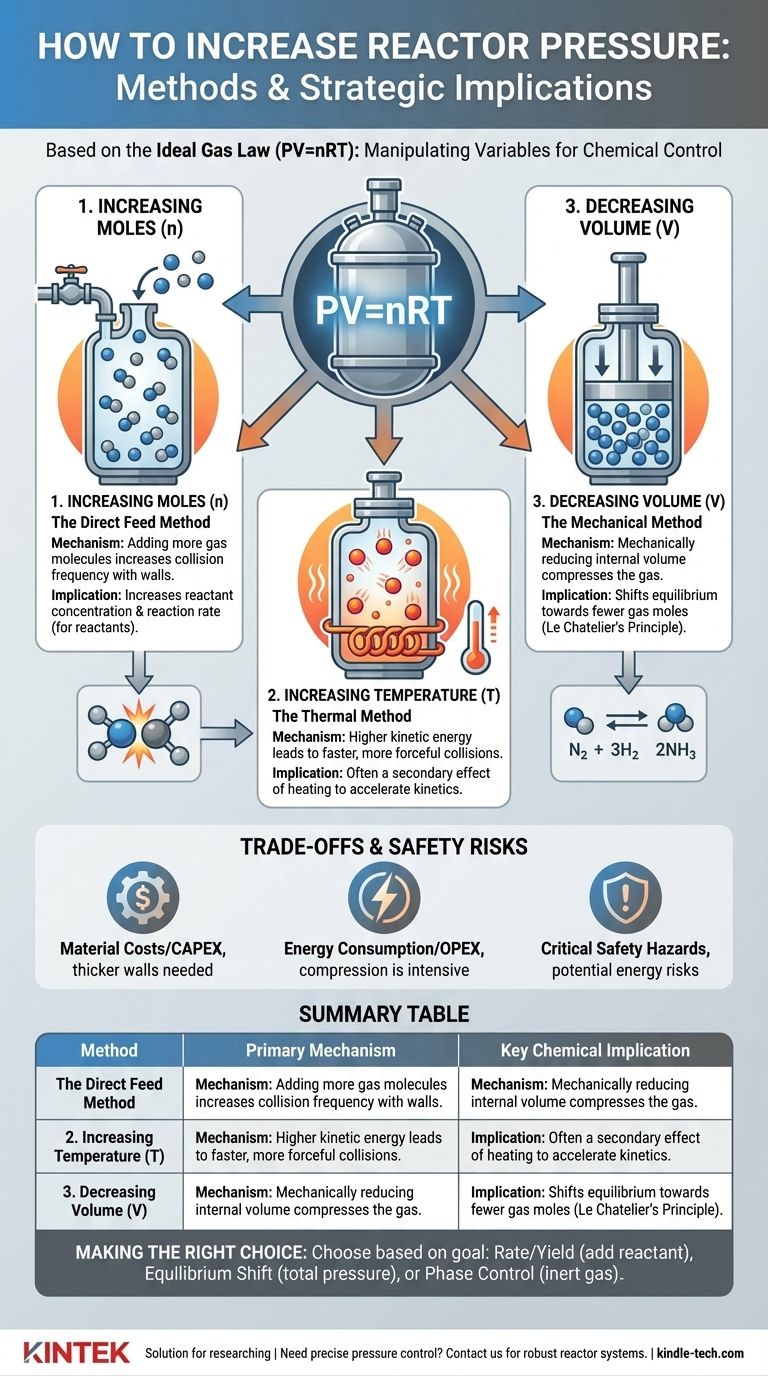
In chemical engineering, increasing pressure in a reactor is fundamentally achieved by manipulating the variables of the Ideal Gas Law (PV=nRT). The most common methods are introducing more material (gas), increasing the system's temperature, or mechanically reducing the reactor's volume. Each method serves a distinct purpose and carries significant implications for the reaction's kinetics, equilibrium, and overall safety.
The core challenge is not simply how to increase pressure, but why you are doing it. Choosing the right method depends entirely on whether your goal is to increase reactant concentration, shift chemical equilibrium, or control the phase of the materials within the reactor.

The Fundamental Principles of Pressure Generation
At its core, pressure is the result of gas molecules colliding with the walls of a container. To increase this pressure, you must make those collisions more frequent or more forceful. The Ideal Gas Law provides the theoretical framework for how this is done.
Increasing Moles (n): The Direct Feed Method
This is the most straightforward approach. By adding more gas molecules into a fixed volume, you increase the number of collisions with the reactor walls, thereby raising the pressure.
However, a critical distinction exists between adding a reactant and adding an inert gas. Adding more of a gaseous reactant increases its partial pressure, which directly increases its concentration and can accelerate the reaction rate.
Conversely, adding an inert gas (like nitrogen or argon) increases the total system pressure but does not change the partial pressures of the reactants. This technique is often used to control phase behavior or for heat management, not to directly influence the reaction rate.
Increasing Temperature (T): The Thermal Method
Heating a sealed reactor increases the kinetic energy of the gas molecules inside. These energized molecules move faster, leading to more frequent and more forceful collisions with the reactor walls, which manifests as an increase in pressure.
This method is often a secondary effect of running a reaction at a higher temperature to increase its rate. The pressure increase must be anticipated and managed as part of the reactor's design.
Decreasing Volume (V): The Mechanical Method
For certain reactor types, pressure can be increased by mechanically reducing the internal volume. Think of a piston in a cylinder compressing a gas.
This method is less common for large-scale continuous reactors but is a primary principle in certain laboratory set-ups, batch processes, and specific types of compressors or engines.
Chemical Implications of Higher Pressure
Increasing pressure is a powerful tool used to influence and control the outcome of a chemical reaction. It is not merely a physical parameter but a key driver of chemical behavior.
Impact on Reaction Rate
For most gas-phase reactions, increasing the pressure by adding more reactants forces the molecules closer together. This higher concentration leads to more frequent molecular collisions, which generally results in a faster reaction rate.
Impact on Equilibrium
This is governed by Le Chatelier's Principle. If a reversible reaction has a different number of gas moles on the reactant and product sides, changing the pressure will shift the equilibrium.
Increasing the pressure will favor the side of the reaction with fewer moles of gas. The classic example is the Haber-Bosch process for ammonia synthesis (N₂ + 3H₂ ⇌ 2NH₃), where high pressure is used to shift the equilibrium toward the product, ammonia.
Impact on Phase Behavior
Pressure is also a critical tool for controlling the physical state of substances. High pressure can prevent a liquid from boiling, even at high temperatures, which is essential for many liquid-phase reactions. It can also be used to liquefy gases for separation or to facilitate reactions that occur at the interface between a gas and a liquid.
Understanding the Trade-offs and Safety Risks
While higher pressure can offer significant process advantages, it comes with substantial costs and hazards that must be carefully managed.
Material and Construction Costs
High-pressure reactors demand thicker steel walls, more advanced alloys, and highly specialized seals and fittings to ensure containment. This dramatically increases the initial capital expenditure (CAPEX) of a project.
Energy Consumption
Compressing gases to high pressures is an extremely energy-intensive process. This translates directly to higher ongoing operational expenditure (OPEX) and can have a significant impact on the economic viability of a process.
Critical Safety Hazards
The single most important consideration is safety. A high-pressure system stores a tremendous amount of potential energy. A rupture or failure can lead to a catastrophic, explosive release.
All high-pressure systems must be equipped with multiple safety layers, including pressure relief valves, burst discs, and rigorous inspection and maintenance protocols to mitigate these risks.
Making the Right Choice for Your Goal
The correct method for increasing pressure is dictated by the specific objective of your chemical process.
- If your primary focus is to increase reaction rate and yield: Directly adding more gaseous reactant is the most effective strategy, as it increases the partial pressures that drive the reaction.
- If your primary focus is to shift a chemical equilibrium: Increasing the total system pressure, either through compression or adding reactants, is fundamental for reactions where the product side has fewer moles of gas.
- If your primary focus is to maintain a liquid phase above its normal boiling point: Increasing the total pressure, often with an inert gas, is the key to creating the necessary process conditions.
Ultimately, controlling reactor pressure is about strategically manipulating the system's thermodynamics and kinetics to achieve your desired outcome safely and efficiently.
Summary Table:
| Method | Primary Mechanism | Key Chemical Implication |
|---|---|---|
| Increasing Moles (n) | Adding more gas molecules | Increases reactant concentration/reaction rate |
| Increasing Temperature (T) | Raising molecular kinetic energy | Secondary effect of heating to accelerate reactions |
| Decreasing Volume (V) | Mechanically compressing the gas | Shifts equilibrium towards fewer gas moles |
Need precise pressure control for your laboratory processes? KINTEK specializes in high-quality lab equipment and consumables, including robust reactor systems designed for safe and efficient pressure management. Our solutions help you achieve optimal reaction kinetics, yield, and safety. Contact KINTEK today to discuss how we can support your laboratory's specific needs.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
People Also Ask
- What are the technical characteristics of PTFE (Teflon) lined hydrothermal reactors? Comparing α-ZrP Synthesis Methods
- Why are high-strength alloy tube reactors critical for HHIP? Ensuring Safety and Purity in High-Pressure Environments
- What is the function of a PTFE-lined hydrothermal autoclave in cys-CDs synthesis? Achieve High-Purity Carbon Dots
- Why use high-pressure reactors for food waste pretreatment? Boost Hydrogen Production Efficiency Today!
- What role does a PTFE-lined stainless steel autoclave play in the synthesis of BiOBr precursor nanosheets?



















