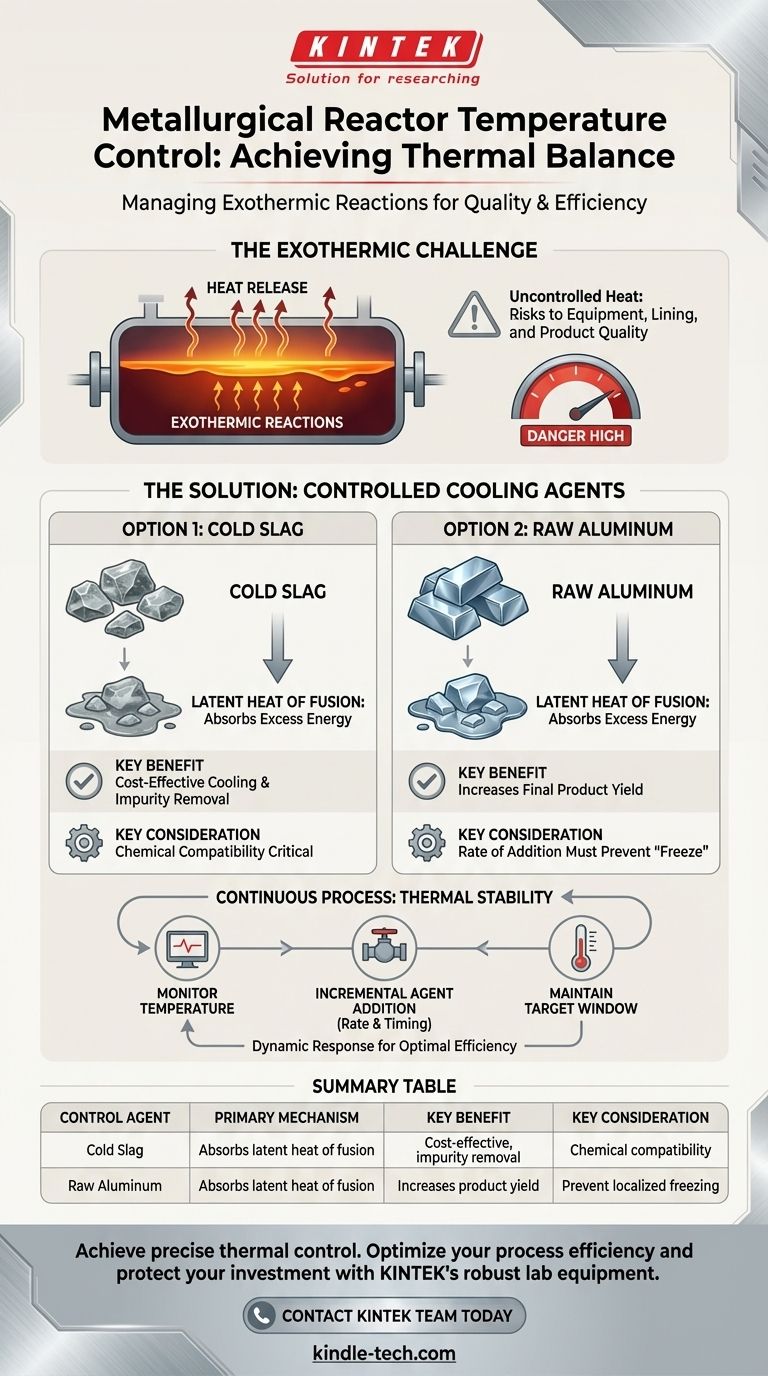
Under normal operating conditions, temperature in metallurgical reactors is controlled through the deliberate addition of cooling agents. These agents, most commonly cold slag or small pieces of raw aluminum, are introduced into the molten bath to absorb excess thermal energy. This action serves the dual purpose of moderating the temperature and contributing to the secondary refining of the metal.
Uncontrolled heat from exothermic reactions poses a significant risk to both product quality and equipment integrity. Effective temperature control is not merely about cooling, but about using the right materials to absorb heat while actively improving the efficiency and output of the refining process itself.

Why Temperature Control is Critical
The Challenge of Exothermic Reactions
Many refining processes, particularly in secondary aluminum melting, are exothermic, meaning the chemical reactions involved generate their own heat. As impurities are oxidized and removed from the molten metal, a significant amount of thermal energy is released.
Without a control mechanism, this self-generated heat can cause the reactor's temperature to rise to dangerous levels. This can damage the refractory lining of the reactor, decrease the lifespan of equipment, and negatively impact the final metal quality.
The Goal: Thermal Stability
The objective is to maintain the molten bath within a specific, optimal temperature range. This stability ensures that refining reactions proceed efficiently, fluidity is maintained for casting, and energy is not wasted. Adding cooling agents is the primary method for achieving this thermal equilibrium.
Analyzing the Control Agents
Using Cold Slag as a Coolant
Slag is a glass-like byproduct of smelting operations. When "cold" (solid) slag is added to the molten reactor bath, it must first melt. This phase change from solid to liquid absorbs a large amount of energy, known as the latent heat of fusion.
This process effectively removes excess heat from the molten metal, acting as a powerful thermal buffer. Furthermore, the added slag can also help absorb additional impurities from the melt, contributing to the refining process.
Using Raw Aluminum as a Coolant
Similarly, adding small, solid pieces of raw aluminum forces the molten bath to expend energy to melt them. This leverages the same principle of latent heat of fusion to reduce the overall temperature.
The key advantage of this method is that the coolant also becomes part of the product. This directly increases the yield of the operation, turning a necessary temperature control action into an opportunity to improve overall material efficiency.
Understanding the Trade-offs and Risks
The Danger of Over-Cooling
While necessary, the addition of cooling agents must be carefully managed. Introducing too much cold material too quickly can cause a localized "freeze," where a portion of the bath solidifies.
This can create operational blockages, make the melt too viscous for proper mixing and pouring, and ultimately halt the refining process, potentially requiring a costly and time-consuming restart.
The Impact on Melt Chemistry
Both slag and raw aluminum alter the chemical composition of the molten bath. The composition of the added slag must be compatible with the desired final product to avoid introducing new, unwanted contaminants.
The rate of addition must be balanced against the rate of the exothermic reactions to maintain both a stable temperature and the correct chemical profile for the target alloy.
The Importance of Rate and Monitoring
Temperature control is not a one-time event but a continuous process. Operators constantly monitor the reactor temperature and add cooling agents incrementally. This dynamic response ensures the temperature remains within its target window without drastic fluctuations.
Making the Right Choice for Your Goal
The selection of a cooling agent depends directly on the specific objectives of the operation.
- If your primary focus is cost-effective cooling and impurity removal: Cold slag is often the preferred agent, as it utilizes a byproduct material to both cool the bath and enhance the refining process.
- If your primary focus is maximizing product yield: Adding small pieces of raw aluminum is ideal because it simultaneously functions as an effective coolant and a valuable input to the final metal bath.
Mastering this thermal balancing act is fundamental to achieving both operational efficiency and high-quality product output in metallurgical refining.
Summary Table:
| Control Agent | Primary Mechanism | Key Benefit | Key Consideration |
|---|---|---|---|
| Cold Slag | Absorbs latent heat of fusion as it melts. | Cost-effective cooling and impurity removal. | Must be chemically compatible to avoid contamination. |
| Raw Aluminum | Absorbs latent heat of fusion as it melts. | Increases final product yield. | Rate of addition is critical to prevent localized freezing. |
Achieve precise thermal control in your metallurgical processes. Uncontrolled temperatures can damage equipment and ruin product quality. KINTEK specializes in supplying robust lab equipment and consumables for demanding refining applications. Our experts can help you select the right tools to monitor and manage your reactor conditions effectively. Contact our team today to optimize your process efficiency and protect your investment.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
People Also Ask
- What is the purpose of using high-purity argon gas in a high-pressure reactor? Ensure Precise Corrosion Test Data
- What roles do autoclaves play in MFI zeolite synthesis? Master Hydrothermal Crystalline Growth
- Why are sealed laboratory reaction vessels necessary in the hydrothermal synthesis of zeolites? Ensure Purity and Yield
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?
- What is the role of high-pressure reactors in the study of alloy oxidation? Essential Tools for Supercritical Research



















